
Is Your Immune-Health Structure/Function Claim Making Implied COVID-19 Disease Claims?

Mitigate the Regulatory Risk by Prioritizing Claim Substantiation
Three current trends in consumer retail behavior reflect the changes in consumer attitudes caused by coronavirus disease 2019 (“COVID-19”): a focus on better (1) self-care, (2) mental health, and (3) convenience in the delivery of healthcare. These consumer trends have created a sales opportunity for manufacturers and brand owners of dietary supplements that support general immune health. However, when marketing teams develop immune health claims, they must account for the increased regulatory risk associated with structure/function claims that may imply a benefit to consumers suffering from COVID-19.
FDA and FTC are Using Warning Letters to Address Misleading Product Claims Regarding COVID-19 Prevention or Treatment
In the past six months, the Center for Food Safety and Applied Nutrition (“CFSAN”) issued eighteen Warning Letters to manufacturers and brand owners notifying them that the United States Food and Drug Administration (“FDA”) and/or the Federal Trade Commission (“FTC”) had reviewed their websites and/or social media and had determined that the companies were selling fraudulent products that bear (1) claims to prevent, treat, mitigate, diagnose, or cure COVID-19 and (2) claims related to the prevention or treatment of COVID-19 that are not supported by competent and reliable scientific evidence.
- FDA and/or FTC reviewed product labels, websites, and social media accounts to determine whether the merchants misleadingly represented that their products are safe and/or effective for the treatment or prevention of COVID-19.
- The claims identified as “related to” coronavirus prevention or treatment ranged (1) from statements on labels, websites, and social media that overtly mentioned “COVID-19” or “coronavirus” (2) to statements that, in addition to claiming an effect on a normal “structure or function” of the body, implied that the product was safe and/or effective for the treatment or prevention of COVID-19 by describing signs or symptoms characteristic of the disease.
- The Warning Letters communicate specific corrective action and advise of the possible enforcement for noncompliance, including legal action pursuant to the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 355(a) (“FD&C Act”) (i.e., seizure and injunction) and the FTC Act, 15 U.S.C. 41 et seq., (“FTC Act”) (injunction and restitution).
Given the current focus of FTC and FDA on misleading product claims regarding COVID-19 prevention or treatment, it is imperative that manufacturers and brand owners of immune health supplements understand the basic legal requirements for the claims used on their labels, websites, and social media.
Making Implied Disease Claims regarding COVID-19 is an Area of Heightened Regulatory Risk
While express disease claims include an overt mention of a disease, implied disease claims can be more difficult to identify because they (1) describe signs or symptoms characteristic of a diseased state and (2) their intended meaning depends on the context within which the claim is made. FDA guidance provides as follows regarding the importance of context in the classification of an advertising statement as an implied disease claim:
“Other signs or symptoms are associated with a wide range of disease and non-disease states and do not necessarily imply an effect on a specific disease. For example, although ‘improves absentmindedness’ might imply treatment of Alzheimer’s disease and “relieves stress and frustration” might imply treatment of anxiety disorders, both of these signs also are characteristic of non-disease states. So, if there is no context linking them to a disease, they would be appropriate structure/function claims.” …[Conversely] ‘inhibits platelet aggregation’ or ‘reduces cholesterol’ are characteristic signs or symptoms associated with stroke and cardiovascular disease, as well as interventions to treat those diseases. Therefore, any statement made in advertising about the effect of a dietary ingredient on these signs or symptoms of disease would constitute an illegal implied disease claim.” (Emphasis added).
Accordingly, an otherwise properly constructed structure/function claim could be classified as an illegal implied disease claim when the statement implies a benefit to consumers suffering from COVID-19 as a consequence of (1) describing signs or symptoms that are characteristic of both common, everyday upper respiratory tract infections and COVID-19 (e.g., cough, fever, nasal congestion, and runny nose) and/or (2) including context on a label, website, or social media that links the statement to COVID-19.
The following are several examples from the illegal implied disease claims regarding COVID-19 that FDA and/or FTC identified in the Warning Letters issued by CFSAN during the past six months:
- Implied Disease Claim:
“Salidroside – Antiviral Properties. . . Its ability to… reduce inflammation is matched by its consistent antiviral activity with research showing it can combat. . . respiratory viral infections.”
Context: From an article titled, “SUPPLEMENTS FOR IMMUNE SUPPORT – CORONAVIRUS (COVID-19) 2020.”
- Implied Disease Claim:
“Vitamin A is actually a hormone that stimulates the production of proteins called cytokeratins. Cytokeratins form a protective barrier around cells that prevents viruses from entering cells. If viruses can’t enter cells, they can’t multiply. If they can’t multiply, they can’t stimulate the immune system response that causes flu symptoms, including death. . . .”
Context: From a website Q&A response to a question about the impact of COVID-19 vaccines on the efficacy of the supplement.
- Implied Disease Claim:
“Vitamin C at extremely high doses acts as an antiviral drug, actually killing viruses.” … “Vitamin C Is a Vastly Underutilized Antiviral ‘Drug’”
Context: From an article titled “Nutrition and Natural Strategies Offer Hope Against COVID-19.”
- Implied Disease Claim:
“You do not need to take this treatment[] continuously for Virus Issues . . . Earth Tea Extra Strength natural antibiotics [will works] on virus . . . You’ll be back to normal within 24-48hrs. Loss of taste and smell will resume there after or immediately.”
Context: From the company’s website.
- Implied Disease Claim:
“[S]elenium is a potent anti-viral mineral. Selenium facilitates enzymatic processes which enhance immune response to viral infections.”
Context: From the company’s website, under the heading “The Scientific and Clinical Rationale for SAFALAB Corona Prevention 12 Pak Remedies.”
- Implied Disease Claim:
“L-Lysine . . . Helps boost immune system response to fight viruses and bacteria that lead to illness. . . . L-Lysine 500 mg by Ecological Formulas features Lysine as its key ingredient, an essential amino acid that helps the body in fighting viruses ….”
Context: From the company’s website https://www.doctorschoice.org/Corona-Virus_c_381.html.
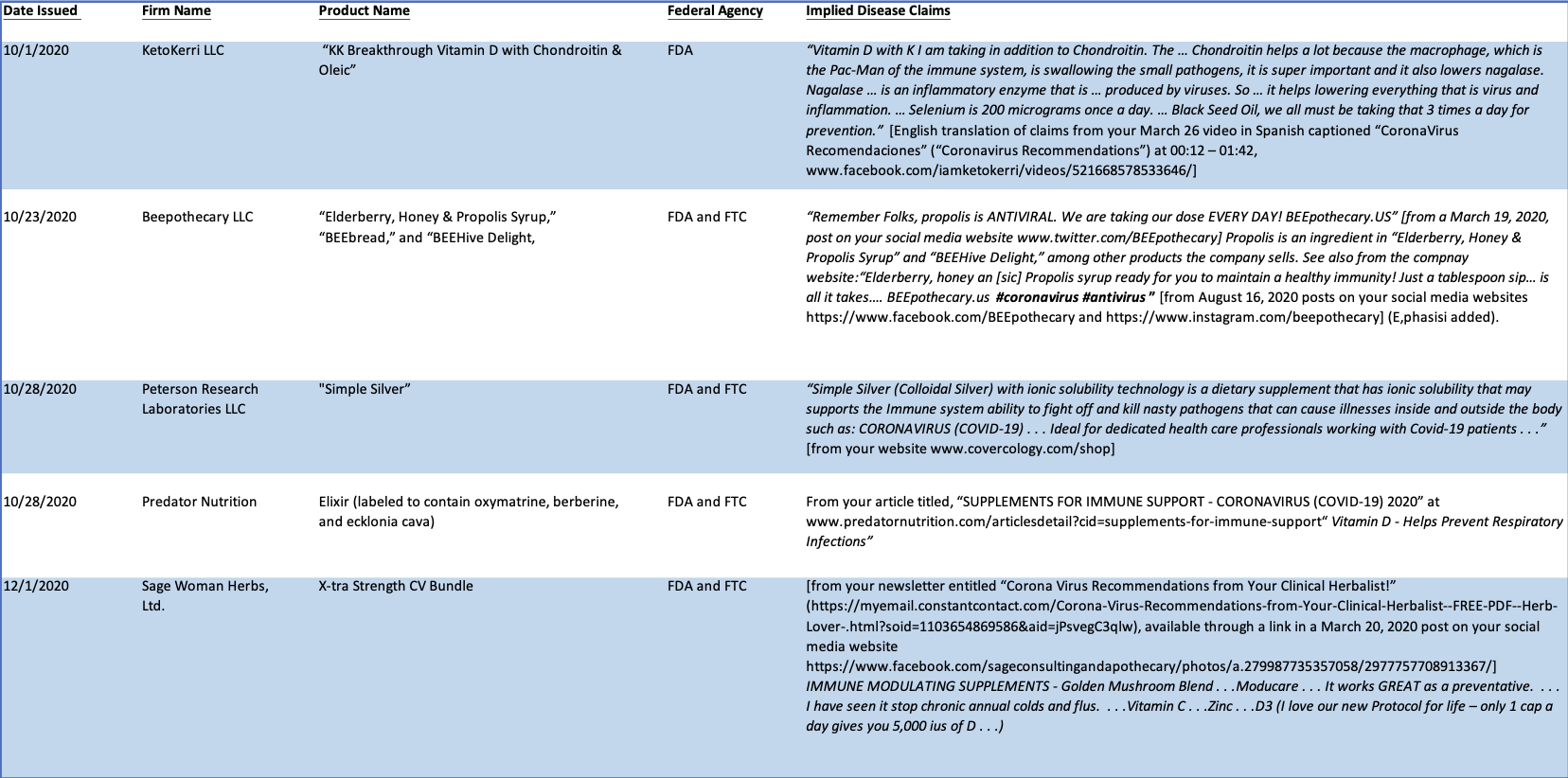
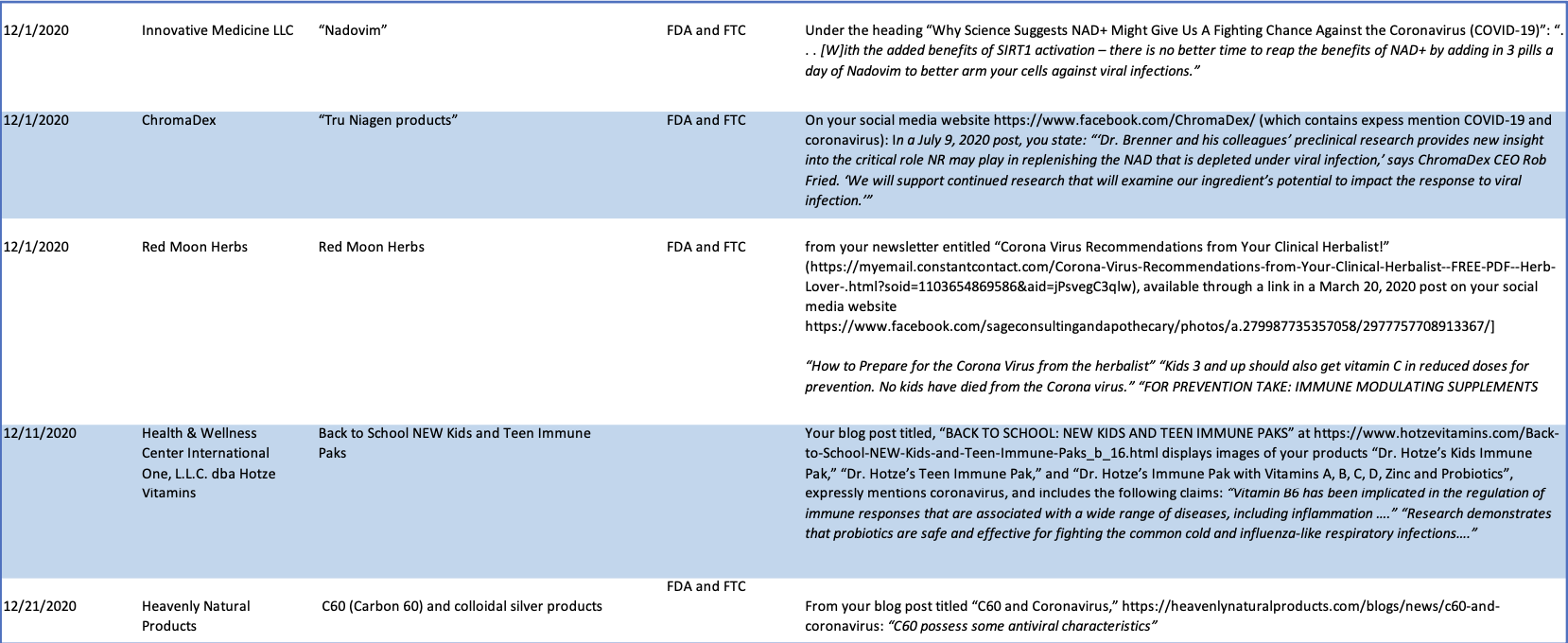
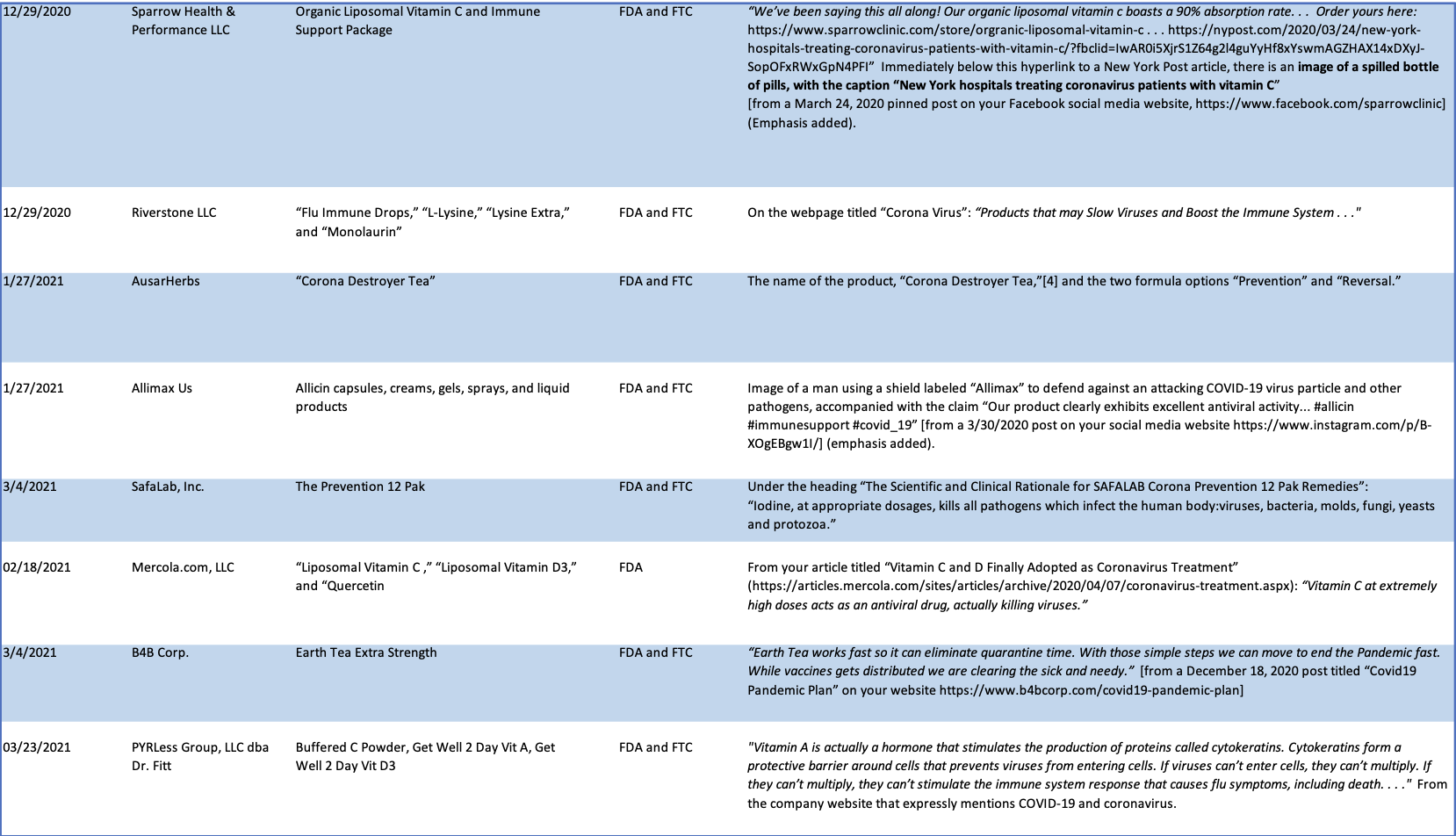
See Chart A (below) for a more fulsome summary of the illegal implied disease claims identified in the Warning Letters issued by CFSAN between October 2020 and March 2021.
Although these statements do not expressly mention COVID-19 or coronavirus, FDA and/or FTC classified them as implied disease claims regarding COVID-19 given the description of signs or symptoms characteristic of COVID-19 and/or the context within which the claims were made that included express mention of COVID-19 or coronavirus. FDA and/or FTC concluded that the statements are not supported by competent and reliable scientific evidence because no study regarding the treatment or prevention of COVID-19 is currently known to exist for dietary supplements that promote immune health.
Prioritize Claim Substantiation to Mitigate Regulatory Risk of Implied Claims Regarding COVID-19
If elements of an otherwise legal structure/function claim regarding general immune health also imply that the product may be beneficial to a consumer suffering from COVID-19, then manufacturers and brand owners must substantiate, with well-controlled human clinical studies, both the structure/function claim and the implied disease claim regarding COVID-19. However, as no studies on the effect of immune-health supplements on COVID-19 are currently known to exist, the most prudent course of action is to eliminate from labels, websites, and social media any information (e.g., URLs, images, hashtags) that could create evidence of the possible intent to make an implied disease claim about COVID-19.
Given that it is difficult for even medical professionals to discern between the signs and symptoms characteristic of COVID-19 and the common, everyday symptoms of sinus or upper respiratory tract infections, the most prudent course of action is to ensure that the design of the scientific evidence used to substantiate the statements on labels, websites, and social media is precisely aligned with the differentiated benefits of the supplement. A claim that a dietary supplement promotes or supports general immune health should be substantiated by scientific evidence that empirically demonstrates the differentiated benefit in healthy populations that bear the same characteristics as the target consumer.
Although this solution appears straightforward, in practice achieving precise alignment between the study design of scientific substantiation and advertising claims can be a difficult task. As a former General Counsel of a vertically integrated manufacturer and owner of powdered dietary supplement, natural care, and wellness brands, I have significant in-house experience with the realities of the advertising claim development and substantiation process.
- Often the Quality Team (which is responsible for claim substantiation) receives pressure from the executive team, business managers, sales, and marketing to quickly substantiate proposed claims so that new products can be launched on a predetermined time schedule.
- Many times, the Quality Team does not participate in the claim drafting process and is asked to substantiate draft claims that are preliminarily approved by the revenue generating side of the business.
- In this context, the Quality Team performs a review of the existing scientific literature, and often identifies misalignments between the design of the existing studies and the express and implied meanings of the proposed claim.
- In my experience, the area of the greatest risk of misalignment between study design and proposed advertising claims is where existing studies are based on a population that is not the same (in all respects relevant to the proposed claim) as the population that will be consuming the product. For example, a study involves young adults, but the product’s claims involve conditions seen only in the elderly.
- Also, I have experienced misalignments between the extent and nature of the effect achieved in the relevant studies and what a claim conveys to consumers. For example, a proposed claim may state that a dietary ingredient will yield a “fast-acting” effect, but the studies do not address a time frame within which the population studied experienced such results.
- Given the pressure to move efficiently towards the launch of a product within a predetermined timeline, the Executive Team may choose to use the available studies that provide the best fit, notwithstanding the identified misalignments. The executives make a risk/return assessment, labels are approved, and the product goes to market.
Ideally, the literature review process would serve to narrow and focus the possible differentiated benefits of the product to those that are empirically observed in a population that is the same as that which will be consuming the new product. Only after a proposed product’s benefits are conformed to reality would it be prudent to begin drafting claim language. However, the claim language is often the first deliverable that is achieved because of time constraints and the push and pull of the competing objectives of the internal stakeholders. Also, crafting claim language on a separate project tract than substantiating the claim increases the likelihood of misalignments that increase the risk that FDA and/or FTC will find that claims are misleading because of a lack of adequate substantiation.
In sum, mitigate this regulatory risk by prioritizing the claim substantiation process and rejecting proposed immune health claims that are not precisely aligned, in all respects relevant to the proposed claims, with the scientific studies that are proffered to substantiate them.
Contact one of YK Law’s experienced FDA and FTC regulatory compliance attorneys to perform an assessment of the adequacy of the scientific substantiation of your dietary supplement claims of general immune health (or any other statement that may imply a benefit for those suffering from COVID-19) as well as to assess the regulatory risk created by the language of the claims made on labels, websites, and social media.
Content paid for and provided by YK Law.
Chart A
CFSAN Warning Letters
October 2020-March 2021.
Related Articles

The Magazine
Information
About Us
NOTE: WholeFoods Magazine is a business-to-business publication. Information on this site should not be considered medical advice or a way to diagnose or treat any disease or illness. Always seek the advice of a medical professional before making lifestyle changes, including taking a dietary supplement. The opinions expressed by contributors and experts quoted in articles are not necessarily those of the publisher or editors of WholeFoods.







