Related: FDA Proposes Reorg; Industry Reacts to Impact on Dietary Supplements
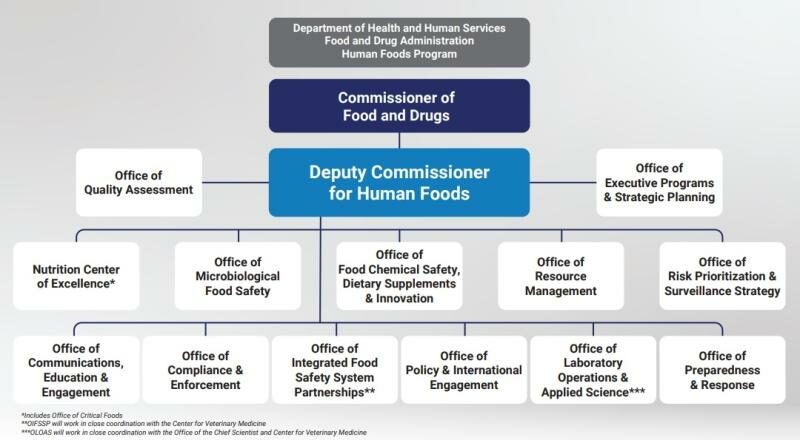
The role of Deputy Commissioner for Human Foods, which Jones is scheduled to begin on September 24, 2023, will report directly to the FDA Commissioner and be responsible for:
* Exercising decision-making authority over all HFP entities when the reorganization is in effect, including related Office of Regulatory Affairs (ORA) activities.
* Providing executive leadership over the entire program as well as over resource allocation, risk-prioritization strategy, policy, and major response activities involving human foods.
* Overseeing the leadership team at the Center for Food Safety and Applied Nutrition and Office of Food Policy and Response. will report to Jones until the proposed HFP reorganization is implemented.
Jones, a former Environmental Protection Agency (EPA) chemical safety office leader, has expertise in issues related to chemical safety and sustainability. Career highlights, as outlined by FDA:
* At the EPA, Jones was a principal architect of the 2016 overhaul of the Toxic Substances Control Act, the first update of that statute in more than 40 years.
* Jones led several national sustainability programs, including the EPA’s Environmental Preferable Purchasing Program and the Presidential Green Chemistry Awards Challenge.
* Jones was a key member of the Reagan-Udall Foundation’s Independent Expert Panel for Foods, which submitted a report on the operational evaluation of the FDA’s Human Foods Program to the agency in December 2022. FDA said this experience makes him "intimately knowledgeable of the agency’s challenges and opportunities, and the panel’s recommendations that the FDA is adopting in its proposal for a unified HFP."
“I’m delighted to welcome Jim to the FDA,” said FDA Commissioner Robert M. Califf, M.D., in the announcement. “His impressive career, extensive leadership experience, and passionate vision for the future of the Human Foods Program make him an ideal selection for this pivotal position. Our proposed reorganization is the largest undertaking of its kind in recent history for our agency. I’m confident that under Jim’s leadership, we will build a stronger organization that will be integrated with other components of the FDA and focused on keeping the foods we regulate safe and nutritious, while ensuring the agency remains on the cutting edge of the latest advancements in food science and nutrition. I’m looking forward to working with him when he joins us next month.”
“I am very excited about the opportunity to serve as the first Deputy Commissioner for Human Foods at the FDA," said Jones. "I had the pleasure of serving on the expert panel that provided operational recommendations for the FDA’s foods-related activities, and I now look forward to helping the agency realize its vision for the proposed Human Foods Program, including carrying out important nutrition initiatives to improve the health of our country,” said Jones. “As a former pesticide regulator, I have a deep understanding of the unique needs of government programs involved in upholding safety of the U.S. food supply, as well as the important role that the agriculture community and state partners play in this paradigm. I am honored to serve the FDA and the country in this new capacity.”
Reactions to on the Appointment Vary
Natural Products Association (NPA): “For more than 30 years, Mr. Jones has held various positions in the U.S. Environmental Protection Agency (EPA) while Mr. Jones is a known commodity in Washington D.C., it is unclear how his experience will benefit the foods program, there seems to be a knowledge gap when it comes to foods,” said Daniel Fabricant, Ph.D., President and CEO of the Natural Products Association (NPA). “At a time when the FDA foods program has a clear accountability gap with Congress, industry, and Americans, as demonstrated by the Reagan-Udall Report, in which Mr. Jones was a panelist, how will a lack of technical understanding for the industries FDA regulates fill that gap? FDA needs to lay out constructive plans to address these deficiencies sooner rather than later. While the reorganization is a significant undertaking, it is not going to have a positive outcome if the new commissioner for foods doesn’t engage immediately with groups like the Natural Products Association to understand the history and specific concerns."
Council for Responsible Nutrition (CRN): “Today’s announcement reflects the FDA’s urgency to improve its oversight of food and strengthen consumer confidence in the safety of these products,” said Steve Mister, President and CEO of the Council for Responsible Nutrition (CRN). "We are hopeful Mr. Jones shares our interests in robust agency attention to dietary supplements as well. We congratulate Deputy Commissioner Jones, and look forward to discussing the many challenges that face our industry, which includes the agency’s prioritization of our needs. We are eager to share with him our concerns and the diverse needs of our membership, and to help him better understand the important space we occupy in the large category of products he now oversees.”
American Herbal Products Association (AHPA): “AHPA welcomes the selection of Jim Jones as Deputy Commissioner for Human Foods,” said Michael McGuffin, President of the AHPA. “We look forward to working with him as part of our ongoing engagement with FDA, and will communicate to him early in his tenure our continuing concern about the potential impacts of the proposed placement of ODSP under a larger office on the regulation of dietary supplements.”
Environmental Working Group (EWG): "EWG applauds Commissioner Califf for appointing Jim Jones as the FDA’s deputy commissioner for human foods," said Scott Faber, Environmental Working Group (EWG) Senior Vice President for Government Affairs. "As a member of the task force appointed to review the infant formula crisis, Jim has a deep understanding of the organizational and cultural challenges facing the FDA’s foods program. As a longtime leader of the EPA’s Office of Chemical Safety and Pollution Prevention, Jim has the leadership skills needed to address those challenges. Jim Jones is the right leader at the right moment for the FDA."