- Can the consumer pronounce the name of the ingredients or excipients?
- Does the consumer know where the ingredient comes from?
- Does the consumer feel better knowing what they’re consuming?
Illustrating the point, Peirce says, “RIBUS, as part of the Clean Label Alliance, sees this definition as an umbrella regarding not only regulations, but industry and consumer terminology. Clean label, we believe, embodies all the certifications (for example, Gluten-Free, Non-GMO Project Verified, Vegan, Organic), applicable statements (for example, Halal, vegetarian), and terms (for example, natural, free-from), that today’s consumer is looking for and answer the above questions.”
Barri Sigvertsen, Senior Manager, Global Innovation Marketing, Lonza Consumer Health & Nutrition, also points to the clean label umbrella, and adds, “We see ‘clean label’ as an opportunity for all stakeholders—consumers, manufacturers and suppliers alike—to reflect on their own version of what the term means to them with integrity; therefore, we believe that transparency and responsibility remain at the core of this trend.” For Lonza, she says, the growth of the clean label trend “has also generated a much bigger conversation, encompassing everything from ingredient sourcing and transparency to quality and responsibility. This market is in synergy with our culture of continuous improvement, both as a responsible business and partner to our customers.”
Len Monheit, CEO, Trust Transparency Center (TTC), agrees that for “clean label” to truly mean anything, transparency is essential. “Clean Label to us means ‘the simplest, least processed set of ingredients conceivable, with complete disclosure about the source, or sourcing, behind those ingredients. And transparency is interwoven by the ‘complete disclosure’ piece above.”
It’s important to recognize that each company is on its own journey in transparency, Monheit adds. “Each must start from somewhere, and there are fewer places to hide your dirty laundry,” he notes. “Fundamentally, you do the best you can, now, and identify your end-goal, and deliver it with transparency.”
Moving toward that end goal, Monheit says, “Developing a strong supply chain and sourcing strategy is the foundation of a successful dietary supplement strategy. FSMA [Food Safety Modernization Act] and best practices will lead to risk management as part of how you source. At the same time, you need to consider how you want to talk about and defend certain ingredients and labels, and plan way up front. Take a look at your products, ingredient by ingredient. Is there a logical, legitimate, authentic story about each of your labels, ingredients, formulations? And, make sure you audit your suppliers to ensure the story you’re telling passes muster.”
For its part in helping companies as they shift to clean label, Monheit says, “TTC has catalogued best practices and has guided companies making the transparency journey, all the way from ingredient sourcing and supply to branded manufacturers. TTC has performed audits and assessments that allow companies to identify and manage the risks and opportunities posed by supply chain partners.”
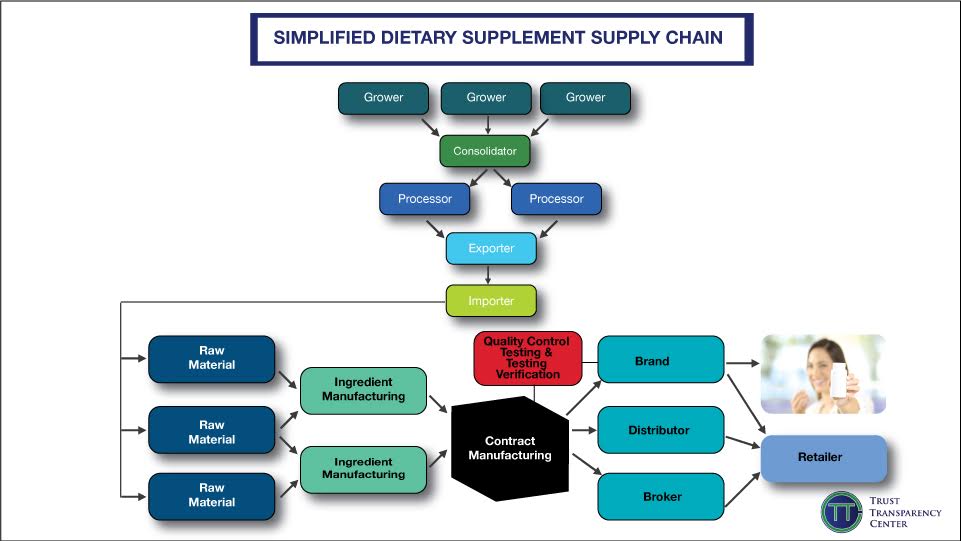
TTC shared two documents that companies can reference: a supply chain map (below) and a checklist. “We recommend companies create their own supply chain map and monitor it regularly,” he adds, “You need to understand your entire supply chain to effectively tell your story and make changes.”
 The Clean Label Alliance, co-founded by RIBUS and Lonza in response to the increasing demand from consumers for cleaner labels on dietary supplements, is another key resource. “Savvy consumers have made the connection between food as nutrition and dietary supplements as nutrition, and desire the same clean label as they have for food with dietary supplements,” Peirce says. “However, as dietary supplement makers transition to clean labels, some are experiencing production challenges. Leaders within the dietary supplement industry joined forces to collectively serve producers seeking to develop or transition to clean label by providing free tech support and problem-solving solutions.”
The Clean Label Alliance, co-founded by RIBUS and Lonza in response to the increasing demand from consumers for cleaner labels on dietary supplements, is another key resource. “Savvy consumers have made the connection between food as nutrition and dietary supplements as nutrition, and desire the same clean label as they have for food with dietary supplements,” Peirce says. “However, as dietary supplement makers transition to clean labels, some are experiencing production challenges. Leaders within the dietary supplement industry joined forces to collectively serve producers seeking to develop or transition to clean label by providing free tech support and problem-solving solutions.”Sigvertsen adds, “We found that one of the key challenges our customers faced when making the transition to clean label claims was navigating this process with, often, a limited knowledge and experience of what it entailed and the wide variety of factors to consider. It was for this reason that we joined forces with dedicated industry leaders and pioneers in the clean label field to create the Clean Label Alliance. With the combined knowledge and expertise of the companies in the Clean Label Alliance, from formulation assistance to equipment handling, supplement manufacturers can more easily bridge the gap and deliver the cleaner solutions consumers are looking for, while also driving forward innovation, quality, efficiency, and effectively communicating what we mean by clean-label.” Interested companies can head towww.cleanlabelalliance.comfor more information, and to fill out a project request form.
For its part, RIBUS is helping brands face the challenge of formulating with new excipients. “The formulators and production teams are familiar and comfortable with traditional, synthetic excipients as they are the industry standard because they work. Our excipients function similarly, but are not identical, so there is a small learning curve. This is one of the reasons why RIBUS started the Clean Label Alliance to help as the industry moves towards clean label.”
Peirce adds that RIBUS produces non-GMO, natural, organic, vegan and gluten-free ingredients for the food, beverage, pet and dietary supplement sectors. “RIBUS’ patented technology and ingredients can help solve production issues while bringing innovation and clean labels to a wide variety of products,” Peirce says. “Regulations and global consumer demands have fueled actions resulting in manufacturers reformulating or creating new clean label products. The RIBUS portfolio offers alternative options to many synthetic ingredients like Silicon Dioxide and Magnesium Stearate.”
To create truly clean label supplement solutions, Sigvertsen notes, brand owners must consider their products holistically. “That is, making sure that not just the ingredient is clean label, but also that the manufacturing processes and the delivery system meet clean label requirements too. All elements work symbiotically, and so it is important that all raw material sources, vendors and practices are clean label to optimize the output. As part of this and our mission to achieve unparalleled leadership in quality and transparency, we have developed a range of ‘Responsibly Made’ capsules and finished products featuring, for instance, our patented Plantcaps capsules. Made from naturally fermented tapioca, Lonza’s Capsugel Plantcaps capsules are a naturally unique, versatile delivery solution. Suitable for both liquid and powder fills, these capsules provide oxygen defense for sensitive ingredients, while also helping to mask taste and odor. Furthermore, our Vcaps Plus capsules are made from FSC-certified Hypromellose (HPMC) and water only, and can now also be tinted using coloring derived from a variety of plant-based foods to create Purple Carrot and new Spicy Yellow and Red Radish options.”
Lonza also continues to expand and invest in its portfolio of science-backed ingredients to complete the consumer experience, Sigvertsen says. This includes the company’s vegan MuscleGuard formula and Oceanix brand, a sustainably sourced phytoplankton ingredient. “Both of these innovative ingredients offer clinically studied benefits for sports performance and recovery, while our ResistAid ingredient, an arabinogalactan for immunity support, is derived from sustainable North American larch trees.”
Communicating with consumers“Today’s consumers are increasingly attuned to the impact that their behavior and purchasing decisions have on their health and that of the planet, and as such are seeking products that reflect these aspirational, values-driven ideals,” says Sigvertsen. “This has been a key driver in the progression of the clean label conversation from its origins, which were based in simplifying ingredient lists and eliminating artificial ingredients to where it is today—that is, a movement that is ultimately driven by a consumer desire to lead healthier lives and make more responsible, sustainable choices. In fact, our research shows that 60% of U.S. supplement users feel guilty about the impact their purchases have on the environment, and 81% would be willing to pay more for a sustainable choice.”
In talks with consumers, it’s important to consider consumer comfort zone and current expectations. As Peirce notes, “We get asked all the time what is wrong with silicon dioxide or magnesium stearate. The truth is, nothing is wrong with using these ingredients. However, more and more consumers are wanting clean labels and to consume ingredients that they recognize. They are more comfortable seeing ‘rice hulls’ on a label than ‘silicon dioxide’ or ‘rice extract blend’ vs. ‘magnesium stearate,’ because they can pronounce the ingredients and they are familiar with rice. As more consumers want and start to require clean labels on their products, RIBUS offers a functional solution for manufacturers and producers.”
Another point to consider when having conversations, Monheit adds, “It’s more than clean label. It’s talking about practices with integrity and authenticity and what goes into your products and how your suppliers are chosen is a part of that. Manufacturers need to get better at talking about that entire narrative—and they are—and then retailers need to be able to articulate that really well in store and make the brand and brand story come to life. It’s also important to think about it from a multi-media perspective—retailers should share stories in their circulars, shelf talkers, staff educators, social media, etc.”
On the horizonWhat’s next for the clean label movement? Peirce says consumers will look beyond the product, and that the demand for product transparency will continue to rise.
To that, Monheit adds a caution: “We see stratification. There is already a minimum threshold, tolerance and expectation set that consumers have of their brands, including supplements. Where this falls down category-wise is the awareness of where raw materials come from and how they are processed—consumers don’t understand that part. This includes the synthetic bio/fermentation story, the natural/synthetic story, and in some cases, processing conditions and extraction solvents. These are vulnerable areas as we seek increasingly to tell an authentic story, and it could all implode. There will certainly be a reckoning.”WF










