What stands out in your mind as the biggest issue affecting the natural products industry in 2015? Supplements safety is likely a major contender.
2015 saw a push from the U.S. Food and Drug Administration (FDA) to stop companies from selling certain ingredients like BMPEA; HBO’s Real Sports with Bryant Gumbel tried to raise questions about “dangerous workout supplements”; Senators Richard Blumenthal (D-CT) and Dick Durban (D-IL) attempted to use the Department of Defense 2016 bill to limit the military’s access to supplements; and Senator Claire McCaskill (D-MO) questioned the sale of certain “dubious dietary supplements.” What’s more, the year rounded out with several federal agencies announcing sweeping actions “to stem the tide of unlawful dietary supplements being sold to consumers nationwide.”
While some efforts were based on true and pressing need, many were not; they grabbed the media’s and consumers’ attention, but have not had a clear impact on safety, experts say.
Unfortunately, many actions falling into this category were those belonging to the person who made the most concerted effort to “rein in” the supplements industry throughout 2015: New York Attorney General (NYAG) Eric Schneiderman. His actions were arguably the most aggressive, the broadest and the deepest felt in the natural products industry this past year.
For nine months, his investigation was responsible for occupying the most time and attention of industry companies and interested groups. As more and more negative headlines broke, some even attributed fluctuation in supplements sales at some stores as shoppers’ response to his accusations.
Given the huge effect he made on this industry, WholeFoods Magazine believes he is the 2015 Person of the Year. But the story doesn’t end there. The efforts of several individuals in setting the record straight about his probe into this industry are incredibly deserving of our attention and gratitude. While many believe Schneiderman missed the mark on improving supplements safety and furthering transparency, these individuals contributed to it by giving consumers complete and accurate information about the industry, products and testing methods in question.
This piece will chronicle Schneiderman’s actions and highlight what the industry has to learn from the individuals who were most vocal in taking down his claims.
Problem One: Methodology and Transparency
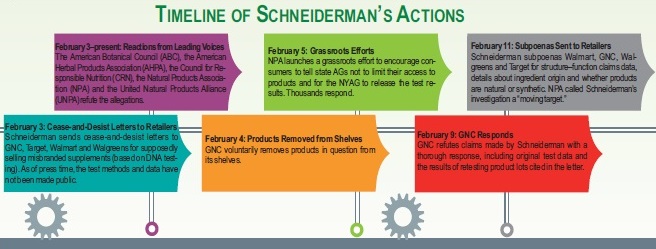
Schneiderman’s initial cease-and-desist letters to GNC, Target, Walmart and Walgreens were based on DNA testing of herbal extracts, a methodology that was refuted by experts. While groups like the American Botanical Council (ABC), Austin, TX, did not criticize the technology itself, they raised a red flag saying it is thoroughly inappropriate to base a legal investigation off this method when it is used alone on most finished herbal products, especially those that are extracts.
Using the tool in this manner, says Mark Blumenthal, ABC’s founder and executive director, “almost ensures that it’s going to have negative results or at least doubtful results.” On the other hand, DNA testing of herbs can be useful before extraction, for certain supplements and along with other established analytical methods.
Pointing out this subtlety was an important contribution of ABC very early in this discussion and helped shape the dialogue about the limitations of this type of testing for botanical extracts. Of note, in 2011 and 2013, ABC released extensive peer-reviewed articles about the benefits, features and appropriateness of using DNA-based analytical methods for botanicals. “It’s another useful tool in the toolbox,” Blumenthal states.
This wasn’t the only problem experts found with the methodology: the testing lab at Clarkson University in Potsdam, NY, had no expertise in botanicals testing, and ABC pointed this out. “You are relying on the expertise of people who doubtfully had any expertise at all in this particular area,” Blumenthal states.
As Schneiderman’s investigation evolved, it appears that the AG agreed with Blumenthal about the inadequacy of the testing lab. Seven months later, he announced that DNA testing of devil’s claw, this time by the esteemed New York Botanical Garden, showed evidence of mislabeling throughout the marketplace.
Blumenthal was also very critical of the Clarkson results from a statistical standpoint, namely an 80% failure rate. His group noted early on in this discussion that the investigators should have taken a step back, recalibrated their machines, checked for contamination and sought verification from a second or even a third lab to confirm the test, using proven compendial methods.
The NYAG’s office did neither any of these things nor backed down from his investigation into this industry. Rather, he escalated his efforts, as is evidenced by the nine-month timeline presented in this piece. Moreover, the complete results of his tests have never been made public, despite consumer outcry for them. For all these reasons, numerous industry voices believe there is a true danger to Schneiderman’s investigation of the supplements industry. “Is that the way we want our public officials to act?” Blumenthal asks. “He’s basically running an ends-justifies-the-means operation, and we were critical of that because we don’t think that’s responsible or ethical.”
This is a dangerous precedent, many feel. “At this point, we believe they know that they were wrong, and they deflected the issue to say, ‘It doesn’t matter what the results of those tests are. What matters is that this is an industry that has problems,’” says Loren Israelsen, president of the United Natural Products Alliance (UNPA), Salt Lake City, UT.
Meanwhile, the UNPA made a huge contribution to the conversation by collecting more than 500 samples of the original products to organize its own legitimate testing of the products in question and making the results public through members of its science and technology group.
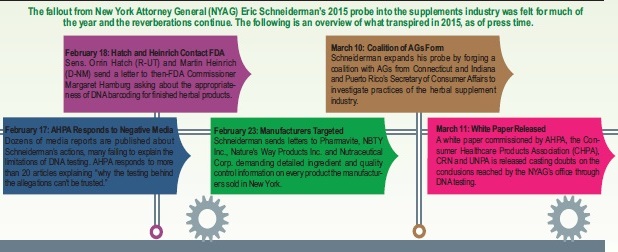
Questionable Outcome
The NYAG’s office believes its investigation has made shoppers safer (see sidebar on page 22). Interviewees disagree.
Israelsen points out that the very GNC products that Schneiderman forced off shelves in New York are already back on the market, without any changes to the formulations. “If Schneiderman’s results were correct, that never could have or would have happened, which means that the results have to be wrong. And, not a little bit wrong; completely wrong,” he states.
In agreement is Daniel Fabricant, Ph.D., CEO and executive director at the Natural Products Association, Washington, D.C., who says, “I don’t think he was out, in any way shape or form, to [improve consumer safety].”
If the NYAG was concerned with safety, says Fabricant, he would have selected more critical issues to deal with and would have filed lawsuits against unscrupulous companies selling illegal products. “The AGs should be there to deal with real consumer threats and real issues,” he believes. “Are there high-priority issues like spiked supplements that FDA and FTC can’t get to because of resources? If there’s a manufacturer or distributor in the state of NY with those sorts of issues, that would be an excellent use of resources by an AG.”
Had Schneiderman consulted one of the groups interviewed for this piece about where to begin an investigation on supplements safety, they likely would have pointed him to some of the known trouble areas—not the herbs and companies he targeted. Instead, the NYAG’s team picked out herbs without much in the way of adulteration issues like valerian root, ginkgo biloba, garlic, echinacea, ginseng, St. John’s wort and saw palmetto.
Meanwhile, in the September probe of devil’s claw, the NYAG’s office went after an herb that is far from the top of the popularity list, and then cited it for being “mislabeled” because it simply has two recognized species that can be substituted under the same common name.
Says Blumenthal, “He could have really done something significant in the matter of targeting real problems that are currently happening in this industry.” Herbs like ginkgo and garlic, he believes, do not qualify.
Steve Mister, president and CEO of the Council for Responsible Nutrition, Washington, D.C., agrees, stating, “[Consumers] didn’t need to be protected against those products. Those products were safe from the get-go.”
Very similarly, Fabricant points out that the NYAG’s office went after companies that were not risky and have never had a problem complying with federal good manufacturing practices. What these retailers all did have are big names yielding big publicity, though. Says Fabricant, “What it says to me is that we need to do a lot better job communicating the value of third-party certification to really clarify the fact that companies are not only compliant, but some firms take steps to go above and beyond to be even more transparent and add value that way.”
As for what interviewees would have placed squarely in the AG’s target, Mister says he would have recommended Schneiderman look at products that contain illegal drugs, anabolic steroids, synthetic stimulants—“things that are clearly illegal and pose a real safety concern for consumers.”
Mister adds that Schneiderman could even have gone to FDA for advice about which lawless companies deserve prosecution for illegal supplements, having done legitimate testing of its own using validated methods. “These are people who are knowingly violating the law,” says Mister.
Conversely, Mister says that the interagency (involving FDA and others) sweep of illegal dietary supplements was a far more effective strategy for improving safety. (see page 8). “They went after companies that had an open disregard for the law, rather than go after mainstream companies in a calculated attempt to try and get lots of publicity for the AG,” Mister says.
Israelsen says he would have told the NYAG about “manufacturing systems, quality systems and certifications…a lot of effort has gone into that on the part of FDA and legitimate industry players.” The reason being, Israelsen says, is so that he can better understand the full spectrum of dietary supplement safety, which includes identity, safety and profile of ingredients coupled with a high-quality manufacturing process (like, say, that of GNC) paired with a well-constructed label and proper substantiation.
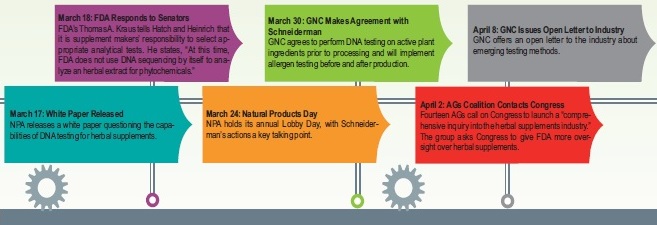
DNA Testing in the Limelight
There may have been a very tiny silver lining to the NYAG’s actions: it re-emphasized that companies should always have the issues of adulteration and quality control front of mind. Says Blumenthal, “There’s no question that he’s influenced this industry in a way that is probably tectonic, which on some levels can be considered a positive thing.”
He says his group and its partners at the American Herbal Pharmacopeia have tried for years to increase awareness that intentional product adulteration needs to be addressed. He calls it “the number one problem in the herbal industry. Not just in the United States, but also internationally. It’s a global challenge.”
While ABC has made headway, he says that Schneiderman very easily got the word “adulteration” on the front page of The New York Times above the fold and, more importantly, made many company executives reevaluate their testing protocols.
It has also raised visibility about the possibility of DNA testing for botanical testing. UNPA, in fact, hosted a two-day summit devoted to the topic in early November. Israelsen said experts tried to answer the question of whether DNA testing is really ready for prime time, and what are its strengths and weaknesses.
Israelsen said it was important for the industry to “vet this very carefully” with the help of “some of the best minds in the world on this” so that companies could have some consensus on the science. “If the industry reacted to say DNA testing is not legitimate in all cases, that would be construed as we’re anti-science and we don’t know how to test products and that we’re trying to deflect the issues,” he states. He believes the UNPA conference represents the most careful examination of DNA testing on botanicals that’s been done to date.
The key conclusions drawn, says Israelsen, were that, “Yes, there’s strong interest in it. Yes, there’s more to learn. No, it’s not ready for prime time. Those that adopt it should do so with great care and understand its limits. They also need to understand that if you don’t control the conditions under which you do the testing, the chances of a mistake go up significantly.”
These may be viewed as “positives” in a way, BUT—and that’s a colossal-sized BUT—those interviewed here say the NYAG should have gone about his investigation completely differently. Says Blumenthal, “Schneiderman’s investigation forced a conversation within the industry that is useful to have. But there are so many other ways that that conversation could have been initiated.”
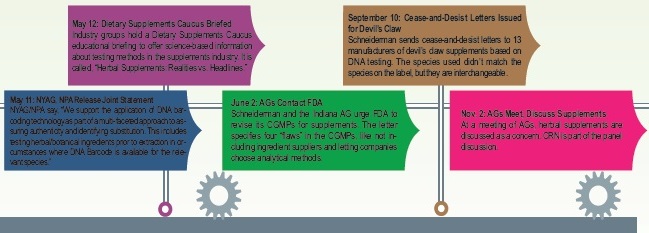
Communication Is Key
It’s unlikely that the NYAG, or most mainstream media outlets, will correct the record on what supplements experts believe are huge errors. Israelsen points out that there’s a true danger to this, since “others that take an interest in this will have an incorrect record to start with, and it only gets worse from there.”
To prevent this from coming to fruition, everyone interviewed for this piece reported that dealing with this crisis was the single most time-consuming endeavor their group dealt with in 2015. Israelsen goes so far as to say his group “communicated more actively and more frequently and with more depth on this than any other issue that we have ever addressed.” The efforts of all those interviewed for this piece shaped the conversation in terms of explaining the limitations and problems with Schneiderman’s investigation.
Starting days into the crisis, for instance, CRN was a strong voice pushing back with scientific evidence for why the test results were wrong. “I believe we were relentless in representing the industry’s view and making sure the consumers and the media knew there was another side out there, that they weren’t getting the whole story from the AG’s office,” states Mister.
Mister says CRN worked around the clock to make this happen. “The staff at CRN took this personally and we were so tenacious to make sure someone was going to cover the industry side to this and the AG got it wrong,” he states.
Groups served as experts for the media, and many responded in writing to negative news articles. For instance, the American Herbal Products Association, Silver Spring, MD, responded to more than 20 media outlets within a week of the initial cease-and-desist letters.
There was also a concerted effort to reach out to state AGs, which is critical given the power and reach the Schneiderman’s actions. While there may not be a direct cause-and-effect relationship, Fabricant believes the NYAG’s investigation has “inspired other state AGs.” In October 2015, for instance, the Oregon state AG Ellen Rosenblum brought a lawsuit against GNC for supposedly selling spiked supplements.
With an eye on the future, several trade associations say they are working with state AGs in new ways on the industry’s behalf. CRN, for instance, has reached out and built relationships with other state AGs to “give them good information about the industry and answer their questions.”
The fruit of this labor is coming to bear, as several AGs are now using CRN as a resource. For instance, on the morning of the interview for this piece in mid-November, CRN had already received two inquiries that day from AGs regarding industry issues. “We’ve built really strong relationships with some of these AGs,” Mister says. “It’s had the effect of reassuring them that this industry has quality players in it, and that we’re regulated by the federal government, and that we want to do what’s right for the consumer.”
The reach of state AGs is somewhat scary since they have subpoena power, the power to levy large sanctions on companies and the ability to prosecute criminals, Mister points out. AGs that don’t use these tools properly to protect consumers can do a lot of harm.
But this doesn’t mean the industry needs to avoid AGs altogether or see them as adversaries.
“On the one hand, it’s a very serious matter when an AG decides to target your company,” says Mister. “On the other hand, there are ways to constructively engage with those AGs before an investigation. And there are ways not only for us, but also for our companies to engage with these state AGs during an investigation so that it doesn’t have to be so adversarial.”
To this point, CRN hosted a webinar in December to offer members information about how to deal with state AGs if one were to come asking for information with a letter of inquiry.
|
Viewpoint From the NYAG’s Office WholeFoods Magazine held a brief interview with the NYAG’s office. Here is what Damien LaVera, communications director, had to say about the investigation. That is why the office started investigating the herbal supplements industry and conducted DNA barcode testing on popular herbal supplements sold by GNC, Target, Walmart and Walgreens…In September, the office sent cease-and-desist letters to 13 manufacturers of Devil’s Claw supplements, which are marketed to arthritis sufferers…NYAG reached an agreement with a 14th manufacturer, Nature’s Way, in which the company publicly committed to employ DNA barcoding across its herbal products line and ensure that its devil’s claw products are manufactured and labeled properly. Our efforts have also significantly increased awareness over the need to enhance oversight over the herbal supplements industry. On February 4, 2015, in response to our investigation, Senators Richard Durbin and Richard Blumenthal called on the FDA to launch a nationwide investigation of the supplement industry. We are also leading a bi-partisan coalition of 14 AGs with Indiana AG Greg Zoeller calling for a Congressional inquiry into the herbal supplements industry. In June with AG Zoeller, our office sent a letter urging the FDA to enhance its oversight of the dietary supplements industry. |
|
Moreover, Rend Al-Mondhiry, CRN’s regulatory council, joined about 80 assistant state AGs as a panelist at the National Association of Attorneys General Fall Consumer Protection Meeting in St. Louis, MO. “She was asked to come and present the industry’s side of the issue,” Mister explains. “When you do things like that and you show a willingness to engage and have dialogue with the state AGs, that builds your credibility with them. And they realize this is not an industry that is trying to avoid scrutiny.”
And, one cannot forget improving relationships with the NYAG. Fabricant and the NPA forged a smart relationship with the NYAG early on in the crisis to ensure accurate information about the industry was provided and available to Schneiderman.
On May 11, Fabricant and Schneiderman issued joint statement representing a distinct change in the NYAG’s verbiage: “While no single test or technology alone can provide complete confidence to consumers, we support the application of DNA barcoding technology as part of a multi-faceted approach to assuring authenticity and identifying substitution. This includes testing herbal/botanical ingredients prior to extraction in circumstances where DNA Barcode is available for the relevant species.”
Of note, six months later, Fabricant reported that NPA leadership is still meeting with the NYAG’s office. This underscores some of the group’s unique abilities to navigate the political landscape, especially given that two former FDA officials (Fabricant and Corey Hilmas, M.D.) work for the group. Says Fabricant, “I’m not going to say what we did yielded a perfect result and that we got everything we wanted, but I certainly think we had an impact and we’ll continue to.”
Fabricant believes meeting with the NYAG’s office is well worth the effort. “A lot of times what happens in these scenarios is that people start out and they don’t know about the other side. They don’t know about the industry. They don’t know what it is they’re actually interested in, so we’re able to educate them on many things about the industry—what it is and what it isn’t,” he states.
NPA has been very politically active during the crisis on a number of fronts, including spearheading a grassroots movement, demanding transparency from Schneiderman’s office. Within days of the initial cease-and-desist letters, NPA called on all industry members and consumers to email, call and tweet the NYAG’s office, and demand that he release the study data. A microsite was established with easy tools for consumers to use to make these communications. These efforts generated thousands of emails to the NYAG in support of dietary supplements.
“We also hit him on social media, which I think is important for the industry,” Fabricant states. “It’s truly one of the first times we’ve seen social media play a role in terms of advocacy and political operations.”
Moving Forward
While as of press time, the NYAG has not made any actions directly questioning industry products after the devil’s claw letters, it would be a mistake to think that NYAG’s office has forgotten about dietary supplements. “I don’t think we’ve seen the end of this,” Mister stated.
For instance, there’s some unfinished business regarding the original cease-and-desist letters sent to four retailers. GNC responded immediately and worked with the NYAG to get the original products back on the shelves. But the other retailers—Walmart, Target and Walgreens—have not reached a settlement with the AG. These stores simply stopped selling the products named in the letters in New York. “At some point, we might see some sort of a settlement,” Mister says.
More unfinished business could happen in a similar vein as we saw with devil’s claw. This herb is certainly not the only plant with two or more species recognized as being substitutable under the same common name. Could Schneiderman find other herbal supplements with similar “mislabeling violations”?
Israelsen says that the NYAG’s new collaboration with the New York Botanical Garden might also give clues for what could be coming down the pipes in the future. He says that the New York Botanical Garden had conducted testing on devil’s claw not long before the September letters were sent. One might guess that some of the other herbs they have investigated recently could be a roadmap that Schneiderman may follow.
Says Israelsen, “I really hope that rather than just loading up the same letter and changing the names to a new plant, they recognize that they still need to understand how botanicals are regulated and the commercial practice and documentations that go along with it.” WF
Published in WholeFoods Magazine January 2016










