In June, we chatted with John J. Cannell, M.D., about the optimal dietary intake of vitamin D. This month, we will discuss the safety of vitamin D.
Dr. Cannell graduated with a degree in zoology from the University of Maryland, where he was a member of Phi Beta Kappa. He received his M.D. from the medical school at the University of North Carolina. After a year-long surgery internship at the University of Utah and four years of practicing itinerant emergency medicine, he began as a general practitioner in the coalfields of Appalachia.
Later, Dr. Cannell left general practice and went back to school to study psychiatry. He moved to Atascadero, CA, in the late 1990s and began working as a psychiatrist at Atascadero State Hospital, the largest hospital in America for the criminally insane. There, his long-held interest in clinical nutrition was re-awakened. The further he studied nutrition, the more and more vitamin D3 (cholecalciferol) caught his attention.
As Dr. Cannell began to study the effects of vitamin D, he immediately realized that the recommendations of the Food and Nutrition Board (FNB) of the IOM were placing many Americans at risk. He found that vitamin D insufficiency was common in older adults, even using conservative cutoff points for vitamin D blood levels. Dr. Cannell was left wondering whom he should believe: Nature or the FNB? In 2003, he recruited professional colleagues, scientists and friends for a board of directors and took the steps necessary to incorporate The Vitamin D Council as a tax-exempt, nonprofit, 501(c)(e) corporation.
In September 2006, Dr. Cannell’s seminal article, “Epidemic Influenza and Vitamin D” was published in the journal Epidemiology and Infection. The article presented a revolutionary new theory on vitamin D’s link to influenza and was co-written with some of the world’s top vitamin D experts.
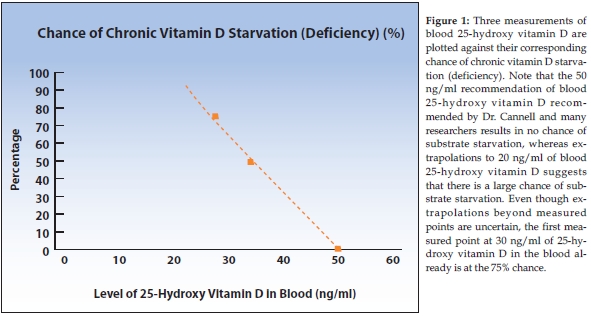
Last month, we discussed how and why many vitamin researchers conclude that the best way to determine vitamin D adequacy is to measure the amount of 25-hydroxy vitamin D in the blood. An intake of 5,000 IU of vitamin D/day will generally lead to a 25-hydroxy vitamin D level of about 50–70 ng/ml, although that amount varies considerably from person to person. A study done by Professor Robert Heaney, M.D., of Creighton University showed that if one’s 25-hydroxy vitamin D level was 30 ng/ml, his/her vitamin D level was zero.
At around 30 ng/ml of 25-hydroxy vitamin D, about 25% of people started having detectable vitamin D levels in their blood. At 35 ng/ml of 25-hydroxy vitamin D, half of the population will also have detectable amounts of vitamin D in their blood. At 50 ng/ml of 25-hydroxy vitamin D in the blood, everyone has some detectable vitamin D in their blood, with more stored in their muscles and fat.
That means that if your blood level of 25-hydroxy vitamin D is 50 ng/ml or more, you will not have chronic substrate starvation. If your level is 35 ng/ml, you have a 50% chance of having chronic substrate deficiency. If it is 30 ng/ml, you have a 75% chance of having chronic substrate deficiency. The results of Dr. Heaney’s study are graphed in Figure 1.
Passwater: Dr. Cannell, you and many, if not most, vitamin D researchers are convinced by the body of scientific evidence that 5,000 IU of vitamin D should be the recommended intake for optimal health. Previously, people had been led to believe that only 400 IU was adequate. Now, when people read recommendations for 5,000 IU, they worry that this larger amount may be harmful. Perhaps, 5,000 IU seems to be a large number to them.
Cannell: It is larger than 400, but in the case of vitamin D, 5,000 IU is only an eighth of a milligram of cholecalciferol; that’s only 125 micrograms. Micrograms, not milligrams! There are 1,000 micrograms in a milligram. Perhaps if the dosage was stated in micrograms, they wouldn’t worry.
Passwater: Last month, we discussed that in 1972, the U.S. Food and Drug Administration (FDA) was trying to lower the amount of vitamin D available in supplements to 400 IU because of the FDA’s “concerns” that more would be toxic. How do you respond to those who remark that at 5,000 IU, vitamin D is toxic?
Cannell: They might think it’s toxic, but they can’t find a case in the medical literature in the history of the world that shows it is. They can’t even find a case of where somebody taking 10,000 IU of vitamin D a day—for no matter how many years—caused vitamin D toxicity.
Passwater: That was true in 1972 when the FDA tried to ban any supplement having more than 400 IU (making them available by prescription only) and in 1986 when I reviewed vitamin D safety in this column (1).
Where does vitamin D intoxication begin?
Cannell: Vitamin D toxicity would generally start to appear if someone was taking 50,000 IU a day for many months. Even in that case, 50,000 IU a day won’t be toxic for everybody, not that anybody needs that much. Vitamin D toxicity has never been documented to occur at blood levels less than 200 ng/ml.
When you say toxicity, people have these visions of being in intensive care units with vomiting and heart arrhythmias, and so forth. The first sign of vitamin D toxicity doesn’t mean that at all. It merely means that you feel perfectly fine, but when you have a blood test, your calcium level is slightly elevated. This is what I mean when I say the beginning of vitamin D toxicity. It is asymptomatic hypercalcemia.
At this level, it would take years and years to injure yourself.
One way to think about it is like water. If you go to your doctor and ask, “Doctor, have you ever heard of or seen a patient who has died from drinking too much water?” Virtually every doctor will say, “Yes.” Water intoxication is not uncommon. It is common in psychiatric patients and in long-distance runners who have this huge amount of water in their backpacks and they drink too much. It can cause swelling in the brain, seizures and death. There are hundreds of cases of people dying every year in the United States from water intoxication. However, you will never meet a doctor who has ever seen anybody die from vitamin D intoxication, unless you are at one of three universities that I am aware of that have reported it. It is that uncommon. Very uncommon!
Passwater: The Institute of Medicine (IOM) whose Food and Nutrition Board sets the diet recommendations (Dietary Recommended Intakes) defines its Tolerable Upper Intake Level (UL) as the highest level of daily nutrient intake that is likely to pose no risk of adverse health effects to almost all individuals in the general population. The UL represents total intake from food, water and supplements. Last year, the IOM raised its UL for vitamin D to 4,000 IU, whereas the Vitamin D Council puts its at 10,000 IU.
Cannell: Yes, we say the upper safe limit should be 10,000 IU. People have to understand what that means. The safe upper limit is the amount that anybody can safely take without seeing a doctor and without having any fear that they  will be doing themselves any harm. It is the level that is safe for healthy individuals to take. However, if you have sarcoidosis (a systemic disease that is characterized by the formation of small grainy lumps in affected tissue) and you take 10,000 IU per day, then you may get profoundly hypercalcemic. But, that’s not vitamin D toxicity. The toxicity is from the sarcoidosis, not the vitamin D.
will be doing themselves any harm. It is the level that is safe for healthy individuals to take. However, if you have sarcoidosis (a systemic disease that is characterized by the formation of small grainy lumps in affected tissue) and you take 10,000 IU per day, then you may get profoundly hypercalcemic. But, that’s not vitamin D toxicity. The toxicity is from the sarcoidosis, not the vitamin D.
One good thing the Institute of Medicine did in its 2010 report was to change the UL from 2,000 to 4,000 IU. Vitamin D researchers all around the country had been plagued by the former UL of 2,000 IU as they often could not get approval to use more than 2,000 IU of vitamin D for treatment from the various safety and ethics committees that overlook research. Now, they will be able to see an effect from treatment with 4,000 IU thanks to the higher official UL.
It’s unfortunate that the IOM didn’t raise the DRI significantly. An interesting inconsistency is that it recommends 400 IU for a newborn infant and 600 IU for an adult woman, pregnant or not. Think about it. The IOM is recommending 400 IU for a five- or six-pound infant (80–67 IU per pound) and even for a 200-pound pregnant woman, it is recommending only about 3 IU per pound or about 1/125th the amount. The mother and baby inside the mother need 600 IU and the baby alone needs 400 IU. The IOM is suggesting that the 195 pounds of the mother alone needs only 200 IU of vitamin D, which is about 1 IU per pound. But as soon as the baby is born, the combination needs 1,000 IU of vitamin D. My contention is that if the mother and baby need 1,000 IU as soon as the baby is born, then they also needed it when the baby was still within the mother.
Passwater: The new report made a couple of small strides forward, but I felt the IOM was trying to save face with old recommendations and wanted to take only small steps. I know that a couple of researchers on the committee wanted to take larger steps forward.
Cannell: One of the problems with the structure of the IOM committee is that if you served on the previous committee, you can’t serve on the new committee. This means that some long-time experts in vitamin D research could not be members now. Another problem with the committees is that the dissenting viewpoints are not published. You don’t even know how much dissension there was or how close the votes were. In fact, they don’t pick “vitamin D experts”; they pick “scientists.” Of the dozen scientists on the vitamin D committee, only three had extensive experience in vitamin D research.
Passwater: The Committee needs to take steps that are more in line with the recent steps taken by several editors of medical journals towards full disclosure.
Cannell: Yes, there even was a conflict of interest that has not been widely talked about. One of the scientists on the Board (or Committee) had financial interest in what are called vitamin D analogs. Here’s how the system works. Natural compounds can’t be patented. If one takes the vitamin D molecule and changes it somehow such as adding on another element, one can get a patent on the new variation. All that one has to do is make sure it doesn’t completely lose its effectiveness and that it is not a naturally occurring substance. Then, one can do a study and compare it with a placebo—not natural vitamin D—and obtain a use patent and apply for a new drug. This is what happened with one of the members and his university. They patented ergocalciferol and continued funding the research with this compound they also called vitamin D. The same has been done with analogs of the active hormone, 1,25-hydroxy vitamin D. Instead of studies being funded comparing these expensive patentable compounds (drugs) with inexpensive natural vitamin D against various diseases, they compared these drugs with placebo.
Some members of the Board as well as their scientific advisors would have adverse financial effects if plain, old, inexpensive natural vitamin D was shown to be better than the patented drugs. I know that these are ethical scientists who believe in what they’re doing, but these sorts of conflicts should be disclosed. I didn’t see anything in the IOM report wherein any scientist disclosed any possible conflict of interest.
Passwater: It is a perverse situation. Now that we have covered how vitamin D works in the body and the recommended dosages for optimal health, I want to move on to your research. Let’s take another break and resume next month with your research and lead up to your new hypothesis about vitamin D insufficiency triggering autism. WF
another break and resume next month with your research and lead up to your new hypothesis about vitamin D insufficiency triggering autism. WF
Reference
1. R. Passwater, “Vitamin Safety, Part VII, Toxicity Potential Strong for D,” WholeFoods Magazine 9 (5) 43–44 (1986).
Dr. Richard Passwater is the author of more than 40 books and 500 articles on nutrition. He is the vice president of research and development for Solgar Vitamin and Herb. Dr. Passwater has been WholeFoods Magazine’s science editor and author of this column since 1984. More information is available on his Web site, www.drpasswater.com.
Published in WholeFoods Magazine, July 2011










