Last month, we discussed the tocotrienol form of vitamin E and cancer prevention with Dr. Barrie Tan. We talked about the differences between the tocotrienol and tocopherol forms of vitamin E and that tocotrienols have proven to contain some exceptional properties that are not shared by tocopherols. This month, we will examine additional specific roles of tocotrienol compared to the tocopherol forms of vitamin E with Dr. Barrie Tan, Ph.D. These roles include reducing heart disease risk and controlling inflammation, as well as supporting nerve health and protecting against radiation and bacteria.
Dr. Tan earned his doctorate in chemistry with an emphasis on biochemistry at the University of Otago, New Zealand. He later became a professor of chemistry and food science/nutrition at the University of Massachusetts, Amherst. His research expertise includes lipid-soluble materials such as carotenoids, tocotrienols/tocopherols, CoQ10, omega-3s and cholesterol. He was the first to introduce tocotrienol’s benefits to the nutrition industry. He founded American River Nutrition Inc. in 1998 and developed the first-ever tocopherol-free tocotrienol product derived from annatto beans. Today, the focus of his research is on lipid-soluble nutrients that have an impact on chronic and degenerative conditions. Dr. Tan was elected to be the senior editor of a compilation with a broad focus on tocotrienols, Tocotrienols: Vitamin E Beyond Tocopherols (AOCS & CRC Press; Fall 2012), and was the organizer of the 2nd International Tocotrienol Symposium in conjunction with the 103rd Annual Meeting of the American Oil Chemists’ Society (Long Beach, CA).
Passwater: Originally, tocotrienols were discovered for their cholesterol-lowering properties in the early 1980s. Is there continued research effort in the area of cardiovascular diseases?
1980s. Is there continued research effort in the area of cardiovascular diseases?
Tan: Research on tocotrienol’s effect in cardiovascular diseases is still ongoing. The lowering of cholesterol and other blood fats by the potent delta- and gamma-tocotrienols was tested in cell lines, animals and clinical trials (30), where results varied significantly based on the study design. In cell lines, the mix of delta- and gamma-tocotrienol suppressed regulators of cholesterol homeostasis genes including HMG-CoA reductase (HMGR), the rate-controlling enzyme of the metabolic pathway that produces cholesterol, resulting in the reduction of cholesterol and triglycerides in mice by 28% and 19%, respectively. In an eight-week human clinical study, supplementation of the mixture lowered triglycerides by 28% without a change in cholesterol levels. The lack of cholesterol lowering in these study participants was most likely due to a high-fat diet during the holiday season. A clinical study is now underway at the University of California, Los Angeles (31) to clarify the effectiveness of tocopherol-free delta- and gamma-tocotrienol (50:50 ratio) on blood cholesterol and other heart disease-related lipids.
Passwater: In our last interview you mentioned that tocotrienols are approximately 50 times more potent as an antioxidant than tocopherols. Do you have any updates on tocotrienol’s antioxidant properties?
Tan: The fact that tocotrienol is about 50 times more potent than tocopherol was an original finding of the antioxidant expert Lester Packer, Ph.D., of the University of California, Berkeley (32, 33). We compared tocotrienol’s radical scavenging capacity in skin cells under UVA-/UVB-induced stress. A tocotrienol-only mixture and the tocotrienol–tocopherol mixture were 46 times and 10 times more potent than alpha-tocopherol, respectively.
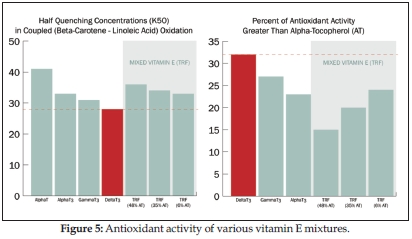
Oxygen radical absorbance capacity (ORAC) is an assay developed by Boxin Ou, Ph.D., and the U.S. Department of Agriculture, and was originally made famous for the measurement of water-soluble antioxidants, such as blueberry and pomegranate, and from here we derive the concept of nutrient-dense “superfruits.” This assay has since been expanded to include lipid-soluble materials. In lipid ORAC studies, delta- and gamma-tocotrienols had the highest antioxidant value of all vitamin E isomers at 5.5 times and three times the potency of alpha-tocopherol, respectively. Interestingly, delta- and gamma-tocopherols were also strong antioxidants (34). In vitamin E mixtures containing both tocotrienols and tocopherols, a higher concentration of alpha-tocopherol was associated with lower antioxidant activity (35) (see Figure 5).
Passwater: What happened in Fukushima, Japan on March 11, 2011 was devastating, and is felt today even more than a year after. The consequences will linger for many years to come. Radiation protection is not a distant or novel idea, but indeed very current.
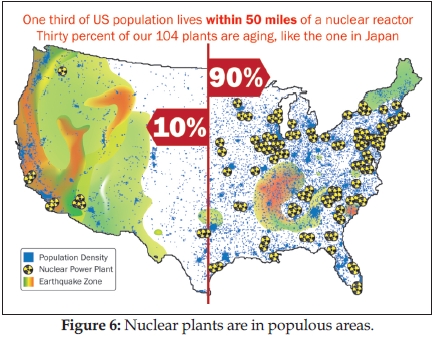
Tan: Quite contrary to being novel, it is indeed a very present and attendant situation in the United States. We have 104 nuclear reactors producing 20% of U.S. power, of which 31 reactors (30% of the total number) are aging like the one in Japan. The U.S. Nuclear Regulatory Commission (NRC) approved all 63 applications for a 20-year extension from a normal 40-year life span, and thus “60 is the new 40” (36).
If you draw a line on the continental United States, 90% of all our plants are on the eastern half of the United States where most Americans live. Furthermore, some of the nuclear reactors are in earthquake-prone areas such as California and South Carolina (see Figure 6). Put squarely together, one-third of the U.S. population (105 million people) lives within 50 miles—the reach of a dirty bomb—of a nuclear reactor.
Passwater: Those are some startling numbers! I understand that advances in radiation countermeasures research are also attributed to tocotrienol’s antioxidant properties, correct? Who is involved with this sort of research?
Tan: The U.S. government. In the past six years, the Armed Forces Radiobiology Research Institute has performed extensive research on tocotrienol as a radiation countermeasure agent (37). Of the tocotrienol isomers, delta- and gamma-tocotrienol are among the most effective radioactive countermeasure agents  (38, 39). Reactive oxygen species and reactive nitrogen species are the primary sources of radiation-induced damage; tocotrienols—as potent antioxidants—are effective radioprotectors, supporting the hypothesis that “strong antioxidants make strong radioprotectors” (40).
(38, 39). Reactive oxygen species and reactive nitrogen species are the primary sources of radiation-induced damage; tocotrienols—as potent antioxidants—are effective radioprotectors, supporting the hypothesis that “strong antioxidants make strong radioprotectors” (40).
However, the amelioration of radiation lethality—which is acute and severe—goes beyond tocotrienol’s antioxidant properties. Radiation’s first line of insult to the human body is bone marrow, which produces blood. Delta- and gamma-tocotrienol display an unambiguous stimulatory effect on hematopoietic (blood-forming) tissue (38, 41), with delta-tocotrienol performing equal to or better than gamma-tocotrienol, restoring the fresh blood supply damaged by radiation insults.
Following total body irradiation of mice, both delta- and gamma-tocotrienol regenerated blood-born cells by increasing the total white blood cell count; in addition, only delta-tocotrienol regenerated lymphocytes. Tocotrienols almost fully restored bone marrow cellularity to normal levels following radiation, while overall cellularity in untreated controls remained depleted (38, 41). In both cases, prophylactic treatment 24 hours pre-radiation was more effective than post-radiation treatment. These results suggest that tocotrienols, especially of the delta- and gamma-isoforms, could be used as powerful radioprotectors for first responders to nuclear fallout areas, radiation workers, and cancer radiotherapy patients.
Passwater: You mentioned earlier that a second edition of Tocotrienols: Vitamin E Beyond Tocopherols will be published this fall, with you as senior editor. What are some examples of the emerging tocotrienol research that will be discussed in this book?
Tan: Various research groups have published studies on tocotrienol’s anti-inflammatory benefits. As discussed in the new book, tocotrienols were shown to decrease NF-kB, a family of proteins that regulate genes critical for inflammation and immunity, and also relevant in chronic inflammation, cancer and aging (42). More simply put, NF-kB is a “master switch” that controls inflammation. Tocotrienol as a potential anti-inflammatory agent was shown not only to affect NF-kB directly, but also its gene products that are involved in carcinogenesis. For example, tocotrienols suppressed COX-2 (a hallmark enzyme present during inflammation), overexpression of which has been associated with cancer, multiple sclerosis and Alzheimer’s disease. Tocotrienol’s suppression of COX-2 did not affect the housekeeping COX-1 enzyme, therefore avoiding serious side effects such as GI and renal toxicities that often occur with nonsteroidal anti-inflammatory drugs (NSAIDS) in this type of application (20).
In general, tocotrienol targets multiple cell signaling pathways, including mono-targeted therapies that inhibit COX-2, and this has been comprehensively reviewed (43). Other research focuses on the effect of tocotrienols in reducing inflammation in experimental mice (44). Researchers demonstrated that alpha-, gamma- and delta-tocotrienols strongly inhibited the inflammatory response by looking at common factors associated with inflammation such as chymotrypsin, trypsin and tumor necrosis factor-a (TNF-a), with delta-tocotrienol being the most effective. Tocotrienols increase the immune system’s ability to fight inflammation, and also enhance the body’s ability to discard unneeded or damaged proteins by modulating large protein complexes called proteasomes. At the same time, tocotrienols induce a hormone and, in turn, produces an anti-inflammatory steroid to block inflammation.
Passwater: Consumers are concerned about chronic inflammation, a condition that seems to increase with aging. This inflammation protection is very impressive. Moving forward, what are some other innovations in tocotrienol science?
Tan: Tocotrienols are currently under investigation for promoting bone mineralization (45) and skin health (46, 47) and increasing longevity (48), as well as counteracting gastric injury (49), metabolic syndrome and obesity (50). Let me choose the last to elaborate on.
Annatto delta-tocotrienol was tested in metabolic syndrome rats fed a high-carbohydrate, high-fat diet. After eight weeks of supplementation, total fat mass and abdominal circumference decreased, while abdominal adipose tissues were reduced, suggesting increased breakdown of lipids and fatty acids. Delta-tocotrienol improved glucose and insulin tolerance, normalized blood pressure and reduced ventricular stiffness. Histological examination showed reduced inflammation.
Passwater: What role, if any, does vitamin E tocotrienol have in nerve health?
 Tan: Tocotrienols, and alpha-tocotrienol in particular, were shown to be effective in treating trauma-induced stroke in animals (51), and a clinical study to investigate this effect further is in the planning stages. Tocotrienols have also been shown to reduce the side effects of diabetic neuropathy (52), and were demonstrated to have a beneficial effect in nerve disorders of the autonomic or involuntary nervous system, in particular familial dysautonomia (FD) (53, 54). In this genetic disorder, children have dry eyes and suffer severe autonomic crises with excessively high blood pressure and heart rate, while enduring violent retching. Children with FD that receive tocotrienol supplementation experience decreased autonomic crisis, and the natural tears have been said to return to their eyes. Most recently, vitamin E deficiency has been implicated in cognitive impairment, where low plasma levels of both tocopherols and tocotrienols were associated with a very high incidence of mild cognitive impairment (92%) and Alzheimer’s disease (94%) (55).
Tan: Tocotrienols, and alpha-tocotrienol in particular, were shown to be effective in treating trauma-induced stroke in animals (51), and a clinical study to investigate this effect further is in the planning stages. Tocotrienols have also been shown to reduce the side effects of diabetic neuropathy (52), and were demonstrated to have a beneficial effect in nerve disorders of the autonomic or involuntary nervous system, in particular familial dysautonomia (FD) (53, 54). In this genetic disorder, children have dry eyes and suffer severe autonomic crises with excessively high blood pressure and heart rate, while enduring violent retching. Children with FD that receive tocotrienol supplementation experience decreased autonomic crisis, and the natural tears have been said to return to their eyes. Most recently, vitamin E deficiency has been implicated in cognitive impairment, where low plasma levels of both tocopherols and tocotrienols were associated with a very high incidence of mild cognitive impairment (92%) and Alzheimer’s disease (94%) (55).
Passwater: Does tocotrienol have any anti-bacterial properties?
Tan: Not exactly, but we have shown that tocotrienols decrease chlamydial infections in vitro by up to 50% (56). We hypothesize that the mechanism at work here is not directly anti-bacterial, but involves cholesterol reduction. Chlamydia are obligate intracellular bacteria that require cholesterol for successful replication within host cells. We believe that the reduction of cholesterol by tocotrienols may prevent the bacteria from invading host cells, hence decreasing their chances for replication.
Other researchers have shown that tocotrienols potentiate the effectiveness of tetanus vaccines (57), and that tocotrienols may be good adjuvants for developing cancer vaccines (58). Tocotrienols were also shown to be immunosuppressive in murine lymphocytes (59), and improved age-related decline of T-cell function in mice (60).
Passwater: Alpha-tocopherol is the vitamin E isoform preferentially taken up by the human body and is most abundant in the blood. Are there any concerns for tocotrienol bioavailability?
Tan: As part of the vitamin E family, tocotrienols are fat-soluble, and are hence absorbed in a similar fashion as fats from food in the gut. It has been shown that tocotrienol is deposited in oil-rich organs. Therefore, enhancing tocotrienol in the blood may become uncertain in meaning, and should not be considered the “yardstick” of measurement. True bioavailability is in tissues, not the blood; the latter is a matter of study convenience.
Passwater: Are you saying that tocotrienol bioavailability is site-specific?
Tan: Yes. In cancer, tocotrienol is recruited and concentrated in the tumor where the cancer is (6, 25). Most compellingly, delta-tocotrienol is recruited directly to the tumor in pancreatic cancer patients (25, 26). In cardiovascular disease, tocotrienol is recruited to the arterial endothelium when atherosclerosis is present. A hallmark of atherogenesis is fatty streak formation in arteries, and begins with the adherence of circulating monocytes—a type of white blood cells—to the endothelium (termed “monocytic cell adherence”). Tocotrienols have been shown to reduce cellular adhesion molecule expression and monocytic cell adherence (61, 62). In particular, delta-tocotrienol showed the most profound inhibitory effect on monocytic cell adherence as compared to tocopherols and other tocotrienol isomers. Delta- and gamma-tocotrienol were 60 times and 30 times more potent than alpha-tocopherol, respectively (63). This phenomenon seems to occur because delta-tocotrienol inhibits the expression of a protein (VCAM-1) that normally causes white blood cells to adhere to the walls of arteries (63).
tumor where the cancer is (6, 25). Most compellingly, delta-tocotrienol is recruited directly to the tumor in pancreatic cancer patients (25, 26). In cardiovascular disease, tocotrienol is recruited to the arterial endothelium when atherosclerosis is present. A hallmark of atherogenesis is fatty streak formation in arteries, and begins with the adherence of circulating monocytes—a type of white blood cells—to the endothelium (termed “monocytic cell adherence”). Tocotrienols have been shown to reduce cellular adhesion molecule expression and monocytic cell adherence (61, 62). In particular, delta-tocotrienol showed the most profound inhibitory effect on monocytic cell adherence as compared to tocopherols and other tocotrienol isomers. Delta- and gamma-tocotrienol were 60 times and 30 times more potent than alpha-tocopherol, respectively (63). This phenomenon seems to occur because delta-tocotrienol inhibits the expression of a protein (VCAM-1) that normally causes white blood cells to adhere to the walls of arteries (63).
In effect, delta-tocotrienol dramatically reduces the “Velcro-effect” of circulating monocytes on the arterial wall, a process known to initiate plaque formation (64). The effects of tocotrienols on atheroma formation (i.e., the accumulation of immune cells differentiated to engulf debris, along with lipids, calcium and connective tissue) have been compared in vivo. Comparison studies on animals investigated the impact of tocotrienol supplementation vis-à-vis tocopherol or non-supplementation. Results to date indicate that animals on an atherogenic diet and given desmethyl tocotrienols—namely delta- and gamma-isomers—had 60% lower plasma cholesterol than the control group, and the size of atherosclerotic lesions was reduced 10-fold. Alpha-tocopherol, on the other hand, had no effect (65). This finding was further corroborated in a similar independent study, where desmethyl tocotrienols inhibited atherosclerotic lesions in hyperlipidemic mice. Atherosclerotic lesion size in mice supplemented with desmethyl tocotrienols was decreased by 42%, whereas with alpha-tocopherol, lesion size was only decreased by 11% (66). Methylated tocotrienols and tocopherols—namely alpha- and beta-isomers—do not have the cardiovascular benefits characteristic of desmethyl tocotrienols.
Passwater: Clearly, tocotrienols recruit to diseased tissues. How about specificity in normal tissue?
Tan: Recently, a clinical study showed that all tocotrienol isomers are actually bioavailable to human tissues (67). Tocotrienols deposited in adipose tissue and the heart, crossed the blood-brain barrier, metabolized in the liver, and were surprisingly low in the blood and skin. My interpretation of the study is that the composition of delta- and gamma-tocotrienol was 1:4—or 20% and 80%, respectively—in the supplement, while tissue distribution in the adipose, blood, skin and brain was reversed at 2:1 or 67% and 33%, respectively. There is strong support that tocotrienols are bioavailable to human tissues, and that delta-tocotrienol’s bioavailability is superior.
Passwater: What are your thoughts on the use of nanoparticles and solubilizers to increase bioavailability?
Tan: Nanotechnology is an engineering field that utilizes super small nanometer particles to enhance functions. In our industry, the goal is to enhance bioavailability of food nutrients with this engineering. Pull back for a moment to appreciate this.
When you make scrambled eggs and pour out a glass of milk, your “beating” and the dairy factory’s “homogenizing” produce microtechnology, making small micrometer particles with other self-emulsifying materials. Nanoparticles are about 100–1,000 times smaller than microparticles—or about 1,000 times smaller than the thickness of a human hair—much like the all-natural LDL and HDL nanoparticles, except that these are made by our liver! LDL and HDL contain fats, cholesterol and nutrients that are ubiquitously delivered everywhere in our body. Synthetically engineered particles will go to places in the body where they have not been before. It is the flipside, the science of toxicity that is unknown and unaddressed.
Here is a concerned remark: “You have a synthetic version of a natural product . . . even though it is only particle size. How do you know where those particles accumulate and how they metabolize?” (68). Very recently, the U.S. Food and Drug Administration issued guidelines to food and cosmetic companies interested in these applications to provide extra safety studies for engineered nanoparticles (69).
There are also pre-solubilized forms of vitamin E out there, but they tend to be in large-size capsules and are difficult to swallow. They contain additives not found in the original plant extracts from where T3 is derived. Oftentimes, tocotrienol is added to a complementary platform with other nutritional ingredients, such as omega-3. Typically, a minimum of 500–1,000 mg of EPA and DHA are needed (70). A tocotrienol–omega-3 combination is wonderful for heart health and perfect for digestion with a meal, but may be too large if a pre-solubilized form of vitamin E is used. I encourage consumers to keep this real simple: take all lipid-soluble vitamins in soft gels (such as A, D, E and K unadulterated) but with a meal, preferably lunch or dinner. This allows the body to do its own solubilization and emulsification.
Passwater: Now to the big question and hot topic. Dr. Tan, you brought up an extremely important topic in our 2008 interview. It concerns the possible interference of alpha-tocopherol—the most commonly used form of vitamin E in dietary supplements— with other vitamin Es, including gamma-tocopherol which predominates in the American diet and the tocotrienols. Let’s devote next month’s column to this important question. WF
Dr. Richard Passwater is the author of more than 45 books and 500 articles on nutrition. Dr. Passwater has been WholeFoods Magazine’s science editor and author of this column since 1984. More information is available on his Web site, www.drpasswater.com.
References
The complete references will follow the last part of this series.
Published in WholeFoods Magazine, August 2012










