My first step was to seek out the world’s best authority in phytoestrogens and cancer. My research indicated that this is Professor Margaret Ritchie, Ph.D., of Dundee University and the University of the West of Scotland. My second step was to visit her laboratory and observe her team of researchers studying P. mirifica’s actions against cancer cells. Professor Ritchie’s publication of a validated database of phytoestrogens blazed the way for many other phytoestrogen researchers to follow.
Dr. Ritchie has a background in pure chemistry from the University of St. Andrews and a passion for the role of phytochemicals on health. Her academic studies include further qualifications and research in the therapeutic use of plant-based bioactive compounds. She was a research Theme Leader in PhytoChemicals at the Bute Medical School, University St. Andrews and has experience in identifying biomarkers of phytochemical intake and performing clinical studies testing the pharmacological activity of herbal medicinal drugs and nutraceuticals.
She was the first researcher in the world to identify biomarkers of phytoestrogen intake and create a validated database of phytoestrogen content of foods. Dr. Ritchie led research at St. Andrews, Dundee and Edinburgh Napier Universities and collaborated in several of the largest European studies investigating the impact of phytochemicals and nutrition on cancer.
She was the Royal Society of Chemistry Education Coordinator for Scotland and advisor to the Higher Education Training Council for Ireland.
Dr. Ritchie has recently taken up new posts with the University of Dundee and the University of the West of Scotland. She is also an accreditation assessor for the Association for Nutrition and a visiting lecturer.
She is currently a member of the National Advisers and Inspectors in Science and a member of the editorial board for the Journal of Nutrition and Food Science.
Passwater: Professor Ritchie, what led to your interest in phytoestrogens?
Ritchie: My major interest has always been in chemistry and while completing my first degree in pure chemistry at the University of St. Andrews, a close relative suffered from cancer. As a result of this personal experience, I decided to use my knowledge of chemistry to try and help in the management of disease, in particular, cancer. By management, I would suggest the prevention as well as the treatment of disease.
I completed further studies after graduating from St. Andrews University and during that time I became fascinated with the actions, properties and potential role of plant chemicals on human health. I learned that plants contain a huge variety of bioactive compounds (i.e., compounds that can promote an effect on the body or tissue or cell). At this point, I decided to find out more about the role of plant chemicals and investigate their properties through research.
After attending a lecture about breast cancer by a surgeon and during subsequent conversations with him and with breast cancer patients, I became aware of increased media attention about breast cancer and research that was being undertaken on phytoestrogens. After following up research leads in the field of breast and prostate cancer, I met staff at Tenovus in Cardiff, Wales, who inspired me to carry out research into phytoestrogens. A key person who trained me in phytoestrogen analysis was Dr. Mike Morton. Dr. Morton and Professor Griffiths (another expert in phytoestrogen research and hormone dependent cancers) were both based at Tenovus, Cardiff.
Passwater: Phytoestrogens seem to be confusing to many health professionals, especially those who do not specialize in them. How long have you been researching them?
Ritchie: I have been researching the properties of phytoestrogens for over 15 years. Although they form the main group in which I have a significant interest and they have been the basis of the majority of my research, I have carried out research involving human trials and other bioactive plant compounds. During my research involving phytoestrogens, I created a validated database containing information about the amount of the common phytoestrogens in food and subsequently, I identified biomarkers of intake using blood and urine samples. I then investigated the effect of intake and exposure of phytoestrogens on the risk of breast cancer and on patients who had been diagnosed with breast cancer.
Passwater: What led your interests to P. mirifica?
Ritchie: During my visit to Tenovus in Cardiff and my meeting with experts in the field of cancer and phytoestrogens, there was a reference to miroestrol, which along with its “sister” compound, deoxymiroestrol, are the most active phytoestrogens in P. mirifica. I was very keen to investigate this herb further. I was also completing several research studies at St. Andrews University and the preliminary results indicated that exposure to phytoestrogens may be beneficial to both breast and prostate cancer patients prior to diagnosis.
Passwater: Okay, let’s start with the basics. What are phytoestrogens?
Ritchie: Phytoestrogens are a group of compounds produced naturally by edible plants. The word “phyto” comes from the Greek word for plants. The term “phytoestrogen” refers to the ability of such compounds to mimic the biological effects of estrogens by binding to and subsequently, activating, the nuclear estrogen receptors.
Passwater: What are estrogens and how do they work?
Ritchie: “Estrogens” refers to a group of hormones with similar chemical structures (see Figure 1) produced in the body from a variety of tissues. Hormones are produced in glands and then travel in the blood to their target tissues. Estrogens are present in both men and women, and they have functions in both, although their levels in women tend to be higher. They have a variety of functions, especially in women during sexual and reproductive development. They can also be known as female sex hormones.
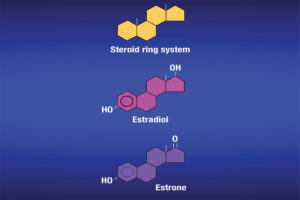
Estrone, estradiol and estriol are the three of the most common estrogens. A fourth estrogen, estetrol, is produced only during pregnancy. It is worth noting that estradiol tends to be present in greater quantities in women of reproductive age. Estriol is the main estrogen produced during pregnancy. Estrone is normally the only estrogen produced after menopause. In other words, estradiol is the more prominent estrogen in non-pregnant females between puberty and menopause; during pregnancy estriol becomes the more prominent; and after menopause, estrone becomes the primary estrogen.
Estrogens are produced from cholesterol. Initially, cholesterol is converted to pregnenolone, which is subsequently converted to progesterone. Progesterone then undergoes conversion into several male hormones including testosterone. Estradiol and estrone can be produced from testosterone. Estriol tends to be produced in large quantities during pregnancy, as I mentioned. Estrogens are responsible for physical changes during puberty in females, which include the start of menstruation and the development of breasts and underarm hair. Estrogens are also responsible for protecting bone health (in both men and women), maintaining cholesterol levels and they can affect mood. The can also affect the lungs and heart.
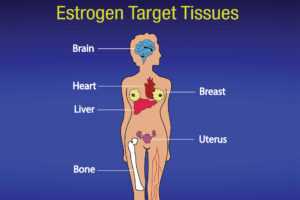
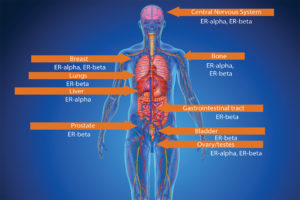
The ovaries, which produce a woman’s eggs, are the main source of estrogens in women. Also, the adrenal glands, located at the top of each kidney, make small amounts of estrogens in both women and men. So does fat tissue. Estrogens circulate in the blood and act nearly everywhere in the body (see Figure 2).
Passwater: What are estrogen receptors and how many types are there?
Ritchie: Estrogen receptors are a group of proteins found within cells and on the surface of cells (see Figure 3). They are receptors, hence their name, that respond to estrogen binding. When an estrogen hormone (i.e., estradiol) binds to an estrogen receptor, it activates the receptor and triggers a response within the cell.
Passwater: How do estrogen receptors work?
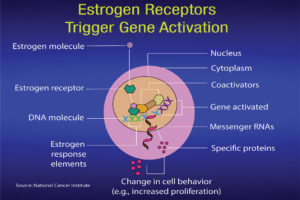
Ritchie: When estrogen binds to the estrogen receptor in the nucleus of a cell, it affects genes involved in development. This, in turn, can form part of an activation signal that will stimulate normal growth or proliferation. The science illustrators at the U. S. National Cancer Institute have drawn figures to help us understand estrogens, estrogen receptors and SERMs (Figures 4a and 4b). The sidebar on p. 54 gives a more detailed explanation for researchers.
Passwater: Back to the basics again. Genes provide the blueprints for making proteins in cells. Each gene makes a specific protein. A gene can be active or dormant. When a chemical activates a gene, it is said to be “upregulated” and it stimulates the production of its protein. Do estrogen receptors work the same in different tissues?
Ritchie: There are two main types of estrogen receptors, namely estrogen receptor alpha (ER-alpha) and estrogen receptor beta (ER-beta).
ER-alpha (ER-α) and ER-beta (ER-β) are found in different proportions in different tissues. ER-α is found in endometrium, breast cancer cells, ovarian stromal cells, and the hypothalamus in females. In males, ER-α is found in the epithelium of the efferent ducts within the male reproductive system. ER-β has been reported as being
present in ovarian granulosa cells, kidney, brain, bone, heart, lungs, intestinal mucosa, prostate, and endothelial cells. The receptors are sites on which estrogens can bind and stimulate a response within the cell. The response will depend on the location of the cell (i.e., in which tissue it is present) (see Figure 5).
Passwater: Discovery of the ER-β by Professor Jan-Ǻke Gustafsson in 1996 while he was at the Karolinska Institute in Sweden dramatically changed our understanding of how estrogens and phytoestrogens work in the body (4).
He is now at the University of Houston. One of the most significant differences between ER-α and ER-β is that ER-α enhances cell division and cell proliferation, a recurring phenomenon in cancer cells. Studies have shown that ER-α is involved in cancer in the uterus and breast, and that the receptor may cause many other negative effects. ER-β, however, is anti-proliferative (5, 6). The estrogen receptors, ER-α and ER-β, oppose and balance one another. While ER-α directs cellular proliferation, ER-β directs cellular differentiation and apoptosis (i.e., programmed destruction of unwanted cells) (7). ER-β is anti-proliferative, in many ways antagonizing ER-α function. Furthermore, phytoestrogens have a binding-preference for ER-β and several epidemiological (correlation) studies indicate a breast cancer preventing effect of this class of compounds (8).
Ritchie: Yes, ER-β may promote anti-proliferative effects. As a result, it may oppose the actions of ER-α in reproductive tissue. This may be useful as some studies have shown that ER-α promotes the growth and proliferation of breast cancer cells. ER-β may also have an important role in the adaptive function of the lung during pregnancy.
ER-β has been reported to be a potent tumor suppressor and as a result it may play an important role in several forms of cancer including prostate cancer. While based at St. Andrews University I was involved with the largest prostate cancer study in Europe in collaboration with the University of Edinburgh where we investigated the effect of phytoestrogens on the risk of prostate cancer.
Passwater: Whoa! May I interrupt you? Perhaps I shouldn’t divert you from the main topic, but a study of phytoestrogens on prostate cancer would be extremely important. Please tell us more about the study.Ritchie: At the time, this was the largest population-based case-control study of diet, inherited susceptibility and prostate cancer in Europe (9, 10). The study was designed to investigate the effect of phytoestrogen intake and serum concentrations of phytoestrogens on the risk of prostate cancer in men aged 50–74 years. Over 800 cases and controls completed the study and the results showed a significant reduction in the risk of prostate cancer with increasing serum concentrations of enterolactone (i.e., a phytoestrogen from lignans). There was also a significant inverse correlation between the intake of soy foods and the risk of prostate cancer. Overall, the study supported our initial hypothesis that soy foods and enterolactone protect against prostate cancer in older men.
Estrogen Receptors: Detailed Description for Researchers
Estrogen mediates these activities via binding to a specific nuclear receptor protein, the estrogen receptor (ER), which is encoded by two genes (ER-α and ER-β) that function as transcription factors to regulate the expression of target genes. On ligand binding, ER undergoes conformational changes and dissociates from the inactive ER-hsp90 complex. The activated ER enters the nucleus as a homodimer or heterodimer, then binds to a specific DNA sequence, the estrogen response element (ERE), and stimulates estrogen-target gene expression. The two ERs have unique tissue distributions and their own sets of specific functions.Passwater: Very interesting, indeed! It was worth interrupting you, but let’s get back to your discussion of the differences between the alpha and beta estrogen receptors. You were explaining how the same estrogen—the same identical compound—can produce different results in different tissues due to the differences in the estrogen receptors in the tissues. It’s not so much the estrogen, but the receptor that is most important. The receptor doesn’t work without the estrogen, but what it does depends on is the type of receptor. Do the estrogen receptors result in different proteins in different tissues or organs?Ritchie: May I be a little technical for a moment?
Passwater: Absolutely! Please do. Some of our readers will appreciate the additional information, while others don’t need any more details than we have already discussed. They can skip ahead to my next question.
Ritchie: OK, I will provide some background here as this may help explain why different proteins result in different tissues. ER-α and ER-β are encoded by distinct genes located on different chromosomes and they have different biological functions. Interestingly, estrogen receptors form dimers (i.e., larger molecules that are made up of both proteins covalently bound together). As a result, the receptors may be made up of ER-α (αα) or ER-β (ββ) homodimers or ERαβ (αβ) heterodimers, which are coexpressed in a number of tissues. In addition, there have been at least three ER-α and five ER-β isoforms identified, which shows the complexity associated with this area of research and why further research is warranted. Both ER-α and ER-β exhibit tissue- and cell-type specific expression and although ER-α and ER-β have overlapping roles, they also have unique roles in estrogen-dependent action within living systems. It has been reported that when both ER-α and ER-β are coexpressed, ER-β exhibits an inhibitory action on ER-α mediated gene expression and, in many instances, opposes the actions of ER-α.
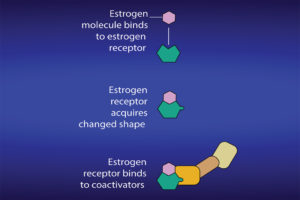
What is significant is the process during which the estrogen binds to the receptor and results in the production of specific proteins. After attachment to estrogen response elements in DNA, the estrogen-receptor complex binds to coactivator proteins causing genes nearby to be activated. The active genes produce molecules of messenger ribonucleic acid (RNA), which guide the synthesis of specific proteins. These proteins can then influence cell behavior in different ways, depending on the cell type involved. In mammary cells, the significance can be cell proliferation, increased cell division, disruption of the cell cycle and checks that take place during cell replication, apoptosis and control of cell death, regulation of cell function, DNA repair and tumor formation.
Interestingly estrogen receptors are over-expressed in around 70% of breast cancers. Different versions of the estrogen response gene have been associated with different risks of developing breast cancer. In tissues with estrogen receptors, the estrogen-responsive genes present and the protein produced may influence the risk and type of cancer.
Passwater: There are various types of phytoestrogens. What are the main types?
Ritchie: Currently more than 300 estrogenic compounds have been identified in plants, the most common of which are isoflavones and coumestans. Lignans have recently been included as phytoestrogens and some flavonoids such as several chalcones, flavanones, flavones and flavonols have also been shown to have estrogenic activity. Herbs such as soy, licorice, red clover, thyme, turmeric, hops, verbena, oregano and damiana are also sources of estrogenic compounds.
Isoflavones occur mainly in one subfamily of Leguminosae, namely the Papilionoidae, although they have been reported occasionally in Compositae and Rosaceae. They were formed about 120 million years ago via the flavone biosynthetic pathway.
Isoflavones were the principal flavonoid compounds necessary for providing antifungal, antibacterial and antiviral protection to plants. Several of these compounds were also potent insecticides with the capacity to mimic estrogen in mammals. Isoflavones constitute the largest proportion of the phenolic antibiotic phytoalexins, compounds that are synthesized only when an infection has started. Isoflavones are closely related to flavones in structure and have similar spectral and chemical properties. The most commonly occurring isoflavones in the diet are genistein and daidzein.
Passwater: Some phytoestrogens are called Selective Estrogen Receptor Modulators? What are SERMs?
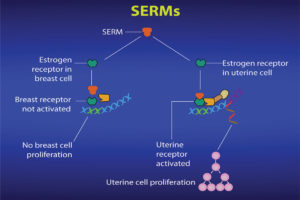
Ritchie: SERMS are chemicals that bind to the estrogen receptor in cells in the way that (endogenous) natural estrogen would bind. Once bound, they may cause a conformational change in the receptor similar to that of an antiestrogen binding. In addition, by binding to the estrogen receptor and preventing endogenous estrogen binding (and promoting an estrogenic response), SERMS can block estrogen binding to the binding site and therefore prevent an estrogenic response. In other words, the cell does not receive the stimulus from estrogen to grow and multiply (see Figure 6).
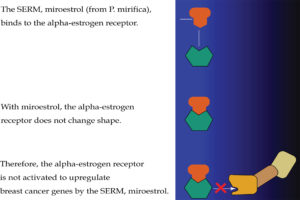
Passwater: How can a SERM be “selective?”
Ritchie: SERMs are “selective” in that they can block estrogen’s action in breast cells and yet can activate estrogen’s action in other cells, such as bone, liver and uterine cells (see Figure 7).
Cells in other tissues in the body, such as bones and the uterus, also have estrogen receptors. Each estrogen receptor has a slightly different structure, depending on the kind of cell in which it is located. So breast cell estrogen receptors are different from bone cell estrogen receptors and both of those estrogen receptors are different from uterine estrogen receptors.
Passwater: Are all phytoestrogens SERMs?
Ritchie: Although there are over 300 compounds that have already been identified as phytoestrogens, there are probably more plant-based chemicals that have yet to be discovered and classed as phytoestrogens. As a result, the potential properties and actions of phytoestrogens are based on current research and published literature. SERMs are non-steroidal agents that bind to the estrogen receptor and promote or prevent a partial estrogenic response. Phytoestrogens are so called as they are plant based compounds that can bind to the estrogen receptor and stimulate an estrogenic response. As several phytoestrogens have undergone research and been shown to demonstrate SERM-like properties it can be assumed that, based on current research findings, most phytoestrogens may behave in a similar way to SERMs. That said, a significant amount of research regarding SERM activity has been carried out using specific isoflavones, lignans, coumestans and stilbenes and the assumption about SERM activity is based on these findings.
Passwater: What are estrogen mimics or estrogen disruptive pollutants and how do they differ from phytoestrogens and SERMs? Can phytoestrogens and SERMs protect from estrogen mimics?
Ritchie: Estrogen mimics or endocrine disruptive pollutants may be described as endocrine disruptors. Such agents may consist of drugs, pesticides, compounds used in the plastics industry and in consumer products, industrial byproducts and pollutants, and even some naturally produced botanical chemicals.
There is some evidence that endocrine disruptors may affect humans and wildlife. The effect on humans could be on reproduction, breast development and cancer, prostate cancer and the response of the brain to hormones. Additional effects on the brain, thyroid, metabolism linked to obesity, and the cardiovascular system have also been proposed. Interestingly, the period that is most critical during development is the transition from a fertilized egg into a fully formed infant. As cells begin to grow and differentiate, balancing the effects of hormones and protein changes is very important. As a consequence, exposure to disrupting chemicals may cause considerable damage to a developing fetus. However due to body weight, the same level of exposure to an adult may have no significant effect.
As phytoestrogens may be able to bind to the estrogen receptor and potentially prevent the binding or an endocrine disruptor, they may be able to reduce or limit the effects of endocrine disruptors. As far as I am aware, there is a dearth of studies reporting a negative impact of high phytoestrogen intake (in mothers) on children.
Passwater: Are phytoestrogens safe?
Ritchie: As with all nutrient and non-nutrient components of a diet, it is important to follow a lifestyle and dietary regime that is balanced. To date, I am not aware of any studies in which exposure to phytoestrogens in humans has caused deleterious health effects. Patients with thyroid problems should consult a health care professional if they wish to increase their intake of specific phytoestrogens.
Passwater: Have you found that P. mirifica affects the alpha-estrogen receptor and beta-estrogen receptor differently? If so, what is the significance?
Ritchie: There is some evidence to show that exposure to P. mirifica may alter the level of expression of both receptors by increasing expression of the beta-estrogen receptor relative to the alpha-estrogen receptor. This finding is based on studies involving cell lines. These findings indicated the significance of the period of time over which exposure took place and the P. mirifica concentration to which cells were exposed.
Passwater: You and your colleagues were researching the effect of P. mirifica on estrogen receptors while I was visiting your lab in 2008. What were your findings?

Ritchie: The findings provided evidence that breast cancer cells (MCF-7 cell line was used) responded to P. mirifica treatment. Two effects were reported: first, the growth of the cells over time and during exposure to varying concentrations of Pueraria mirifica. Another effect that was investigated involved the expression of ER-β relative to ER-α.
The results provided some initial evidence that exposure of breast cancer cells to P. mirifica may increase the ratio of the beta-estrogen receptor relative to the alpha-estrogen receptor over a period of 24 hours and that at higher concentrations, P. mirifica had an anti-proliferative effect on breast cancer cells (11, 12).
Passwater: What is the significance of your findings?
Ritchie: The significance of these studies is that the results were dependent on time and on the concentration of P. mirifica used. It is important to extrapolate the results with care, as in animal and human models, complex physiological processes need to be taken into account when considering the effect on humans.
However, if the expression of the beta-estrogen receptor becomes more prevalent and the ratio of the beta-estrogen receptor relative to the alpha-estrogen receptor is increasing, this might be important because the over-expression of the beta-estrogen receptor has been shown to decrease the transcriptional activation of the alpha-estrogen receptor.
Furthermore, as the beta-estrogen receptor may have anti-proliferative effects and oppose the actions of the alpha-estrogen receptor in reproductive tissue, this may be an important finding on which to base future studies in cancer and breast health.
A feature of research in phytoestrogens (and nutrition in general) has been that duration of exposure and also the quantity used or amount of exposure by an individual seemed to be an important factor in health promotion or disease prevention.
Passwater: Have other researchers followed up on your findings?
Ritchie: An animal study that was carried out and published at the same time reported similar findings. Rats were exposed to increasing levels of P. mirifica over a period of four weeks and similar effects were noted in the ratio of the beta-estrogen receptor to the alpha-estrogen receptor.
Passwater: Where is your research leading you now?
Ritchie: I have been investigating the impact of plant-based products on the immune response and inflammation in human studies while maintaining my interest in phytoestrogen research and nutrition. An ideal study would be to link phytoestrogens with a further study involving the immunological response and investigate the combined effect in humans.
Passwater: Thank you for your very informative discussions, Professor Ritchie. WF

Dr. Richard Passwater is the author of more than 45 books and 500 articles on nutrition. Dr. Passwater has been WholeFoods Magazine’s science editor and author of this column since 1984. More information is available on his Web site, www.drpasswater.com.
References 1. R.A. Passwater, “Menopause and Pueraria mirifica,” WholeFoods Magazine (October 2016), www.wholefoodsmagazine.com/category/ columns/vitamin-connection. 2. R.A. Passwater, “Pueraria mirifica: Just for Menopause or the Herb of the Decade? Part 1. An Interview with Dr. Garry Gordon,” WholeFoods Magazine (Feb 2007), www.drpasswater.com/nutrition_library/gordon_1.htm. 3. R.A. Passwater, “Pueraria mirifica: Just for Menopause or the Herb of the Decade?: Part 2. An Interview with Dr. Garry Gordon,” WholeFoods Magazine (March 2007), www.drpasswater.com /nutrition_library/gordon_2(1).htm. 4. G.G. Kuiper, et al., “Cloning of a Novel Receptor Expressed in Rat Prostate and Ovary,” Proc. Natl. Acad. Sci. USA, 93 (12), 5925–5930 (2006). 5. A.M. Brzozowski, et al., “Molecular Basis Of Agonism And Antagonism In The Oestrogen Receptor,” Nature, 389 (6652), 753-758 (1997). 6. O. Treeck, et al., “Estrogen Receptor Beta Exerts Growth-Inhibitory Effects On Human Mammary Epithelial Cells,” Breast Cancer Research and Treatment, 120 (3), 557–565 (2009). 6. A. Morani, M. Warner and J.A. Gustafsson, “Biological Functions And Clinical Implications Of Oestrogen Receptors Alpha And Beta In Epithelial Tissues,” J. Intern. Med. 264 (2), 128-142 (2008). 7. J. Hartman, A. Ström and J.A. Gustafsson, “Estrogen Receptor Beta In Breast Cancer—Diagnostic And Therapeutic Implications,” Steroids 74 (8), 635–641 (2009). 8. C.L. Heald et al., “Phyto-Oestrogens And Risk Of Prostate Cancer In Scottish Men,” Br. J. Nutr. 98(2), 388–396 (2007). 9. C.L. Heald et al., “Phyto-oestrogen Intake In Scottish Men: Use Of Serum To Validate A Self-Administered Food-Frequency Questionnaire In Older Men,” Eur. J. Clin. Nutr. 60 (1), 129–135 (2006). 10. C. Boonchird, T. Mahapanichkul and W. Cherdshewasart, “Differential Binding with ER-alpha and ER-beta of the Phytoestrogen-Rich Plant Pueraria mirifica,” Braz. J. Med. Biol. Res. 43 (2), 195–200 (2010). 11. S. Ramnarine, J. MacCallum, and M. Ritchie, “Phyto-oestrogens: Do They Have A Role In Breast Cancer Therapy?” Proceedings of the Nutrition Society, 68 (OCE2), Jan 2009 , E93 doi: 10.1017/ S0029665109990462.
Published in WholeFoods Magazine November 2016










