Under the usual guise of protecting the public and the more-honest notion of rationalizing herbal-medicine regulations, the European Union (EU)’s Parliament and Council adopted its Traditional Herbal Medicinal Products Directive (No. 2004/24/EC), or simply the THMPD, in 2004. The THMPD established a regulatory approval process for medicinal herbs within the EU, and it began to come into effect early that same year with a phase-in period to end April 30, 2011. Prior to this Directive, the regulation of medicinal herbs had been left in the hands of the individual EU member states. So, it is easy to imagine the initial delight of the herbal-products industry upon seeing the creation of a new Europe-wide regulatory regime for medicinal herbs that offered the promise of free trade in such herbs through a simplified registration process. 
That was nearly seven years ago; but soon after, the industry’s delight turned into a frown that, for some, has now slipped into outright despair. The promised simplified registration process has become a huge burden for some smaller- and medium-sized companies because of the costs involved in creating a supporting dossier and pursuing registration. Just as disturbing, the regulatory review and approval of the applications and supporting dossiers has so badly lagged that a large backlog is currently overwhelming the system. With the April 30th deadline looming, consumers have been alarmed that their favorite herbs will soon be removed from the marketplace (1).
Registration
So, how is the system supposed to work? Any company selling or wanting to sell herbal medicines* in the EU must now register each and every one of its herbal medicinal products with an EU-member national drug agency. By law, a medicinal product may only be placed on the market in the EU once a marketing authorization in accordance with Directive 2001/83/EC (as amended by Directive 2004/24/EC) has been issued by the competent authority of a member state for its own territory (“national authorization”) or when an authorization has been granted in accordance with Regulation (EEC) No. 2309/93 for the entire community (a “Community authorization”).
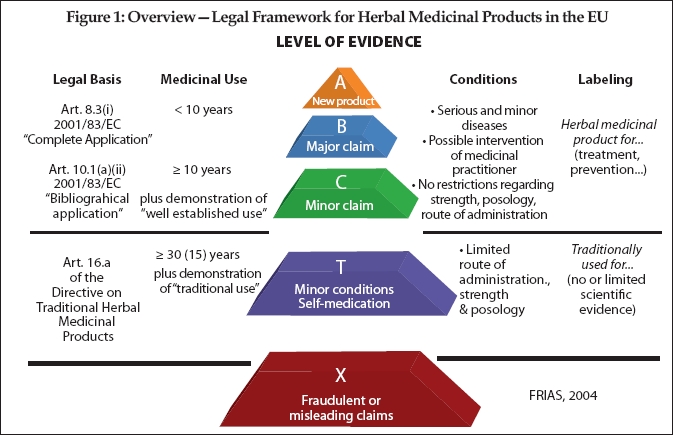
The difficulty of the registration and authorization process is directly dependent upon how traditional or well-established the use of the herb has been. Under the Directive, if the medicinal herb has been in use within the EU for at least 30 years (or at least 15 years in the EU and 15 years outside the EU)—the “30/15 Rule”—then it is likely to be found to be a “traditional herbal medicinal product” (THMP) and the simplified procedure truly is simple**. Registration of a THMP requires general documentation demonstrating by specific references to published scientific literature that the THMP’s ingredient herb(s) have a 30-/15-year medicinal use with recognized efficacy and level of safety. THMP registration costs for a well-prepared application could be as low as $20,000–$30,000, if the required quality documentation is already in existence (otherwise the costs for compiling the application dossier, including stability testing, amongst others, need to be taken into consideration, which could amount to another $50,000–$70,000). Most of the German medicinal herbs would fall into this category, and the German products constitute about 45% of the medicinal-herbal market in Europe.
For well-established medicinal herbs, the registration and authorization process is more involved than for THMP, but more in the nature of the amount of documentation than in kind. The costs for these applications will typically be more than double those of a well-prepared THMP application.
The problem comes with those medicinal herbs that have been marketed within the EU for fewer than 30 years (for THMPs) or even the 10 years for well-established use. These are herbs such as hoodia and acai; and the expense and the trouble involved in pushing through to approval those types of applications for registration will be far greater, not as simple, and will cost somewhere in the range of $100,000–$120,000 each, at a minimum, depending upon the associated claims.
The expense for these non-traditional-use and non-established medicinal herbs is not so much related to the status of the herb (except for when it fails the requirements for both THMP and well-established-use and therefore needs to undergo a full drug approval procedure, in which case the cost could easily reach beyond $500,000 including clinical trials, etc.). Rather, the status depends upon the quality of the documentation that the manufacturer may already have on file (or not). Moreover, some of the requirements are simply very difficult and therefore expensive to satisfy from a technical perspective, especially for complex herbal products. This is where the money goes, and this is why German products have a certain advantage as by German law they have already been treated as drugs and all of the necessary documentation has already been created.
For small companies, and for those medicinal herbs that use new herbal ingredients with no history of use in Europe, this cost is prohibitive. The EU might just as well have outlawed the medicinal herb insofar as its claims are concerned.
Claims Are the Key
Thomas Brendler, one of the principals at Plantaphile Ltd. (www.plantaphile.eu and www.thmpd.eu), a service company with a wealth of experience in pushing through just these sorts of registration applications, has noted, “Everybody can keep their herbal products in the market (THMPD or not), however, associated claims need to be approved (through product license or the European Food Safety Authority). So, while no approval means ‘no claims,’ it does not mean that a product can no longer be marketed.”
And the difficulty in registration and authorization is also directly dependent upon the claim being made. The level of evidence and documentation through clinical trials that is demanded by the EU authorities is illustrated in the accompanying pyramid chart created by the Freiburg Institute for Advanced Studies (FRIAS) (see Figure 1).
Basically, from a regulator’s perspective, medicinal herbs are nothing more than herbal food supplements with documented claims. If you do not make a medicinal claim or an approved food claim, then you are simply selling (or buying) a product as a food supplement, whether it is before or after April 30, 2011.
Here is How it Really Works
Mr. Brendler has perhaps given the most understandable example of how the THMP registration/authorization process works, when he recently wrote (2), “Let’s assume the U.S. company ‘Herbulance’ has an herbal supplement in the U.K. market containing three herbs with an indication for constipation. They could:
(a) Obtain a full drug license (MA or Well-Established Use)—This would require full documentation with adequate proof of efficacy (either through clinical trials or bibliographic evidence). If no clinical evidence is provided, then community monographs could determine such parameters as claims, etc. The product would remain OTC but pharmacy only. This route can be onerous and costly ($150,000 and up).
(b) Obtain a THR (THMPD)—if their herbs meet the requirements (30/15 years of use) and are deemed suitable for THR (unsupervised administration, indication). THR requires full-quality documentation (including stability tests) and suitable evidence for traditional use and safety. Depending upon how well-documented your product already is, this route can cost anything between $30,000 and $100,000, including fees and the like.
(c) Keep it a food supplement and seek an approval for their claim from EFSA (and while EFSA hasn't decided, they can keep marketing their product as before).
(d) Keep marketing their product without a claim (unless EFSA has approved the claim).”
There are, then, a number of choices for manufacturers, not all of them practical; but what seems clear is that come May 1, 2011, there will not be a mass extinction of herbal products from the asteroid impact of the Directive so much as a dearth of health information about a slew of herbal products.
Herbal Medicines, Supplements, Foods & Practitioners
So for purposes of this Directive, remember to distinguish among the different ways in which herbs can be sold—as medicinal herbs, dietary food supplements with authorized claims, dietary supplements without claims, foods such as teas, and those herbs sold through herbal practitioners. The April 30th deadline will only directly affect the first category.
Unfortunately, many herbal practitioners say it is virtually impossible for most herbal medicines to meet the licensing requirements for safety and quality, which are similar to those for pharmaceutical drugs, because of the costs of testing. However, under EU law, statutorily regulated herbal practitioners will be permitted to continue prescribing unlicensed products. So, too, food and food supplements containing herbs will not be affected either by this Directive.
The Downside
Besides the enormous costs that will be inflicted on companies during increasingly tough economic times, important health information will disappear from those products that companies could not afford to register. Indeed, the products themselves will disappear if they are not re-labeled and marketed as food supplements or sold through health practitioners. Consumer choice, as it always does when government regulations are heaped upon industry, will shrivel.
And with the passage of the Nutrition and Health Claims Regulation (EC Regulation No 1924/2006), those medicinal herbs that are repackaged and offered as dietary-food supplements face a very restrictive health-claims environment. The silver lining in that cloud, however, is that with the EU’s Mutual Recognition regulation in place, where a food supplement is permitted to be sold in one member country, then it must be allowed to be sold in any of the other member states (unless a country authority can prove that the product is a risk to public health).
Unfortunate, too, is the fact that not a single product used in traditional Chinese medicine or ayurvedic medicine has been licensed. Mr. Brendler explains, “It is true that the regulation does not provide an ‘easy way in’ for new herbal ingredients with medicinal claims (regardless of traditional use outside the EU); however, in my experience, this affects only a small number of herbs. For classic ayurvedic and TCM herbs proof of traditional use is relatively straight-forward as they have been established amongst the ethnic communities within the EU for a suitably long time. The only issue here is that they thus far may not have been adequately documented in terms of quality and safety.”
Bottom-line: choice and information will diminish while product prices for consumers rise.
The Upside
Is there an upside to all of this? The larger players in the medicinal herb market certainly think so, as do the Germans, who come to the table already dressed for dinner. Their competition is still stuck at the curb, trying to hail the cab.
To be fair, the Directive does confer an advantage of consumer assurance since registration carries a guarantee for quality, safety and—to some degree—efficacy. But, is it worth the trade-off? How many consumers will be unable to buy a particular herb because it is too expensive or outright unavailable? Were deaths and injuries from herbs in Europe at so high a level and of such a pressing concern that this expensive regulatory regime just had to be enacted and enforced?
And, in any event, how can slow-moving bureaucrats ever keep up with the logarithmic advances in knowledge in a sophisticated market economy? Even now, EFSA has been so overwhelmed with work that it has postponed the assessment of health claims for herbal products and raw food materials, which means that absent a negative EFSA assessment, the food authorities will put off enforcement actions on food supplements with reasonable claims (3).
What Will Disappear?
Will traditional Chinese medicines and ayurvedic remedies disappear without THMP registration or EFSA approval? As Mr. Brendler has pointed out, no, they won’t, not as long as they are recognized foods and do not make health claims. Yet, the blind spot in the Directive fails to see that these non-European products have every bit as much legitimacy in herbal medicinal use as any European remedy, probably even more. And what about North-American native medicinal herbs? Will European consumers be denied the benefits of these remedies as well? Who will take them to market if they cannot make claims for their beneficial effects? There cannot help but be fewer choices overall after May 1st, even if some of these medicinal herbs slide into the food-supplement category.
The problem really is two-fold: (1) Too much faith granted to the wisdom of European food and drug bureaucrats; and (2) Too little faith in the wisdom of individual consumers. Overarching this problem, however, is the right of consumers to access commercial free speech without undue limitations. Why should promoting a good and healthy product be treated any differently than promoting a good and healthy political cause?
Market forces, rather than political forces, should determine whether herbs and their claims are available or not. All truthful and non-misleading claims for an herbal product should be allowed. A clumsy and slow, even antagonistic, bureaucracy approving claims is not the answer and may even result in more deaths and injuries from people being denied access to healthy products than if courts enforced people’s right not to be defrauded. In the meantime, with the current regime, experts like Mr. Brendler are pushing the envelope to allow as many legitimate claims as possible to be made. And their interactions with the regulators show us what regulators might have in store for us here in America. WF
End Notes
* Herbal medicines fall under the EU’s definition of medicinal products per Directive 2001/83/EC, as amended June 12, 2003. That definition is found in its Article 1 and sweeps within its ambit “(a) any substance or combination of substances presented as having properties for treating or preventing disease in human beings, (b) any substance or combination of substances which may be used in or administered to human beings with a view to making a medical diagnosis or to restoring, correcting or modifying physiological functions by exerting a pharmacological, immunological or metabolic action” and whereby “substance [includes] any matter e.g. vegetable: plants, parts of plants, vegetable secretions, extracts. . .”
**Compare this to a medicinal herb with a “well-established use” where more documentation is required to achieve authorization, but the “well established” use need only cover a 10-year period. New herbal medicinal products (i.e., those having under 10 years of use) have the most strenuous up-hill climb for becoming authorized.
Editor’s Note: This article is intended for information purposes only. Because state and municipal laws vary greatly, as do the circumstances of individual cases, readers are advised to contact an attorney for specific legal advice © Scott C. Tips 2011
A graduate of the University of California at Berkeley Law School, Scott C. Tips currently practices internationally, emphasizing Food-and-Drug law, business law and business litigation, trade practice, and international corporate formation and management. He has been involved in the nutrition field for more than three decades and may be reached at (415) 244-1813 or by e-mail at scott@rivieramail.com.
References
1. H. Stevenson, “Stop the Traditional Herbal Medicinal Products Directive,” October 12, 2010, www.gopetition.com/petition/39757.html, accessed January 7, 2011.
3. E-mail from Thomas Brendler to Scott Tips, dated January 5, 2011, with caution that this is a simplified sketch of the process to provide only an overview.
3. Press release “Food: Commission reviews the progressive adoption of the list of permitted health claims” (09/27/2010), at http://europa.eu/rapid/pressReleasesAction.do?reference=IP/10/1176&format=HTML&aged=0&language=EN&guiLanguage=fr.
Published in WholeFoods Magazine, February 2011










