The related Amazon statement included in the Amazon Seller Central website:
“Selling partners are required to comply with all laws and regulations, as well as Amazon’s policies including compliance and testing requirements”
Additionally, an Amazon spokesperson said:
“We have proactive measures in place to prevent suspicious, noncompliant or prohibited products from being listed, and we continuously monitor the products sold in our stores.”
While both of these statements have elements of truth to them, neither of them is completely true.
Consider the teenager sent to his/her room with the instruction to clean it before being allowed to do anything else. The child goes away and returns, being asked “Did you clean your room?” The answer is always “yes,” but there’s a huge expectation gap and that first pass was a token job to handle the urgency of the situation.
In this analogy, "clean" may be a journey; that is, it will get better over time as expectations are managed and then reinforced. Obviously, in this analogy, the consumer (or even FDA) is the parent while Amazon is the teenager. The FDA is very clear in its expectations and administration of laws and regulations including compliance and testing requirements. Amazon is being truthful in that they have proactive measures in place to prevent non-compliant products to be listed or sold on the Amazon platform. Yet does Amazon comply fully with FDA expectations? Not yet. Is the attempt “good enough?” Also, not yet.
Since dietary supplements are ingestible, the stakes are higher than simply cleaning one’s room. This, in fact, is the reason the FDA has gone to the trouble of defining what responsibility looks like, because harm can occur if the laws and regulations are not met.
Last monththis columnlooked at the compliance highlight checklist compiled by Trust Transparency Center, which describes 17 key components for which all dietary supplement brands are responsible. Within that checklist, only one of the 17 components was addressed by the recent Amazon requirement. Third-party testing is called out as a new Amazon requirement. The 16 other key components are not addressed.
Obviously, there are gaping holes in Amazon’s compliance initiative. The ability to verify compliance may not be easy, but it is possible. In some areas, other ecommerce platforms actually manage to provide more and make it look easier. Are they more committed than to just the appearance? Perhaps.
In fact, Amazon has, in the past, provided more transparency than it does today. In March 2017, Trust Transparency Center (TTC) reported Amazon’s launch of their first dietary supplement brand, Amazon Elements. TTC was excited to detail the increased transparency the market had anticipated, as Amazon Elements detailed Country of Origin, Test Results, and more, including a QR Code on each bottle that led the consumer to a Certificate of Analysis, manufacturing date, contract manufacturer identity, test method and production date.
Sadly, this transparency is gone today. The concept of transparency is still broadly claimed on the Amazon product pages, but all of the tools to support transparency have vanished. Test results, QR Codes, Country of Origin, contract manufacturer information, everything—gone! Amazon provides no explanation why all of that information so proudly detailed previously has been removed. When Trust Transparency Center repeatedly requested explanations, the best it received was:
“All Amazon brands, including Amazon Elements, provide consumers proven reliability of potency, purity and integrity.”
By discontinuing its commitment to transparency (possibly because of the complexity of doing so) Amazon has left the door open for competition to provide much better transparency in the dietary supplement industry.Iherb.comis an equally dominant dietary supplement e-commerce platform in the United States also serving countries around the globe. Iherb.com has picked up the transparency baton Amazon chose to fumble. All dietary supplement products on the iherb.com website include the expiration date of the product the consumer is purchasing, as well as the date the product was first introduced by the manufacturer:
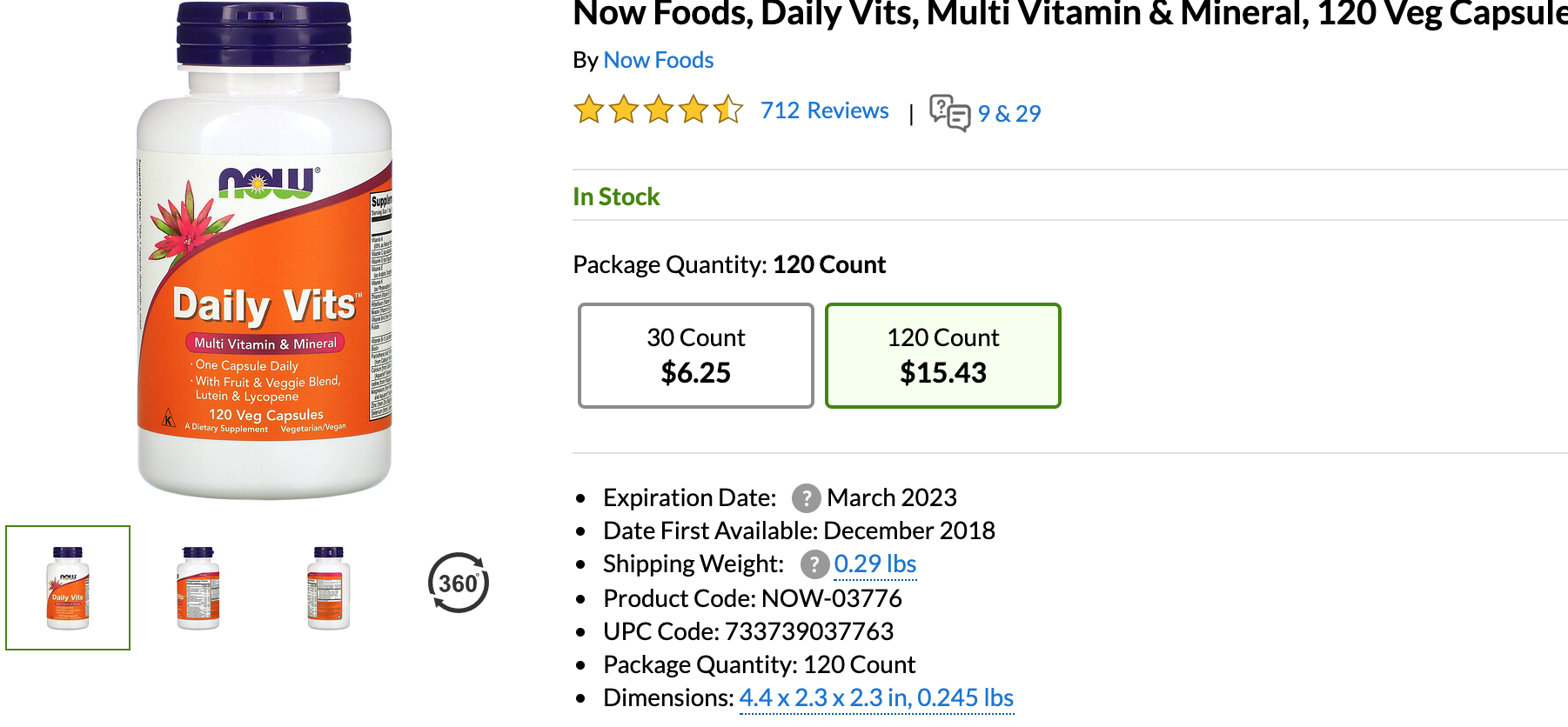
In the case of iherb.com-owned brands, they also offer a stated iTested Certificate of Analysis indicating test results from an independent lab.
Several other online sites provide some degree of transparency by providing images of the labels, manufacturers' websites, etc.The reality is no website can match the transparency, reliability, and authenticity a retail store can provide.By promoting the vetting your organization provides before a product appears on shelf, your consumer is already protected from misinformation or counterfeit concerns. If requested, the brands you carry will provide significant information to support their products. They already provide it to e-commerce companies like Iherb.com because they are required to or the product will not be carried. Trust Transparency Center has conducted several surveys indicating consumers prefer or require more transparency from their brands. Survey your customers and find out what matters most to them and determine the level of transparency you can provide that e-commerce companies probably cannot, or will not, provide.
Also, continue to insist on information from your brands that determine the level of verified compliance with all laws and regulations. By using theTrust Transparency Center Checklistyou can achieve a level of reliability unrealized by any e-commerce offering. By doing so, your organization will provide a more responsible marketplace for dietary supplements in your stores as well as raise the standard for the entire industry to follow.
Note: The views and opinions expressed here are those of the author(s) and contributor(s) and do not necessarily reflect those of the publisher and editors of WholeFoods Magazine.









