
Rich Passwater, Jr., my elder son, has been involved with silicon supplementation and its research for more than 16 years. He is the education director for the Belgium-based dietary supplement research and dietary supplement production company, Bio Minerals NV.
Richard A. Passwater (RAP): Just how is silicon involved with keeping optimal amounts of collagen in both skin and bones?
Richard Passwater, Jr. (RPJr): The important factor is the activation of the enzymes needed to guide the assembly of amino acids to form your own collagen and other proteins of skin and bone. Silicon is needed to activate these critical enzymes and you need to make your collagen as determined by your DNA. The collagen from other animals may supply needed amino acids, but it cannot substitute for your unique collagen.
RAP: Before discussing the details of how silicon activates collagen and elastin production, perhaps we should begin with a quick review of how the body makes them.
RPJr: As with all proteins, the body builds collagen by stringing together single amino acids in a specific order as determined by our DNA. The process starts in the cell nucleus where gene DNA “flows” or is transcribed into messenger RNA (mRNA). mRNA is a template with a series of codons (or triplet codes), each corresponding to a specific amino acid.
After mRNA molecules are formed, they are sent to a ribosome inside the cell. Ribosomes link amino acids together in the order specified by the codons of messenger RNA (mRNA) molecules to form polypeptide chains. Transfer RNA (tRNA) molecules bring the various required amino acids to the mRNA template and begin aligning those amino acids in the proper configuration based on the mRNA codons using a process called translation.
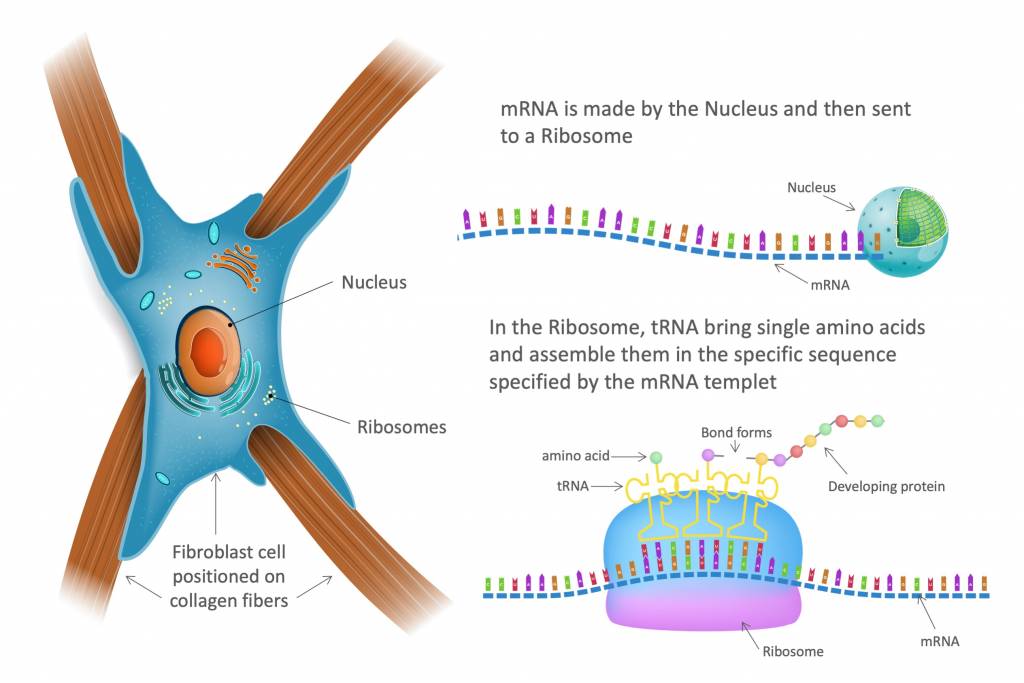
The end result of the transcription and translation process is a single strand of amino acids called an alpha chain. In collagen production, alpha chains generally contain about 17 or 18 different amino acids and are over 1000 amino acids long.
Next the fibroblast cells must transform the various single alpha chains into actual collagen by making a series of adjustments called post-translational modifications. Enzymes perform most of these required modifications.
As an example, prolyl hydroxylase enzymes convert about 40% of the proline in the initial alpha chains into hydroxyproline—a step critical to linking 3 alpha chains together to form a unique triple helix structure. This triple helix structure gives collagen it’s unique strength and flexibility properties.
Somewhat similarly, lysyl hydroxylase converts some of the lysine in the initial alpha chains into hydroxylysine, a process that also strengthens the structure.
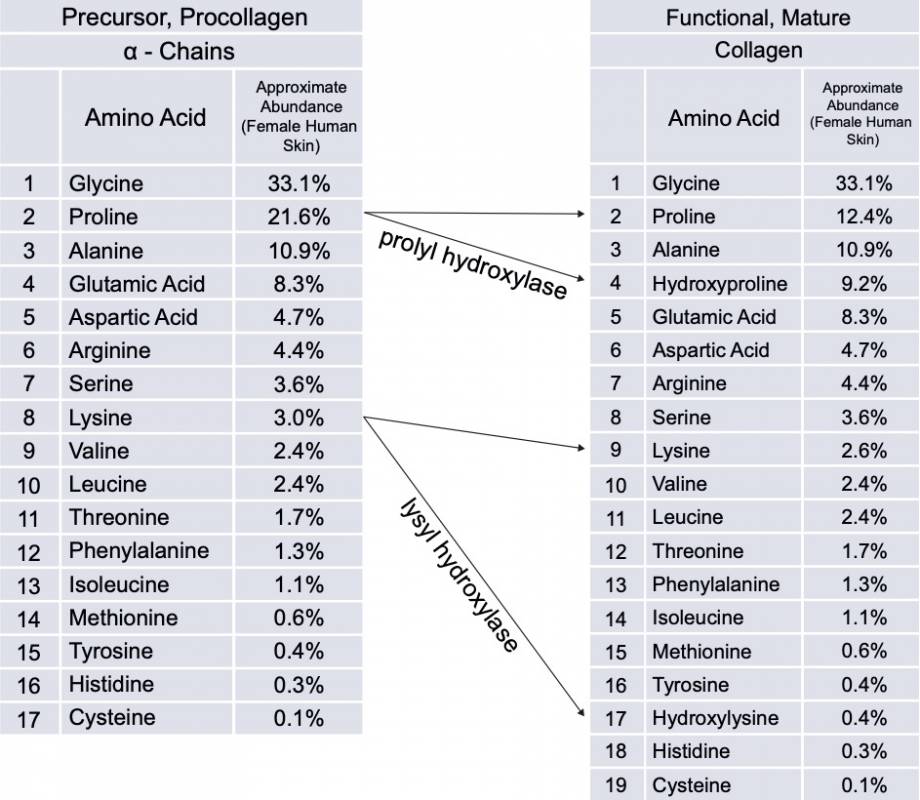
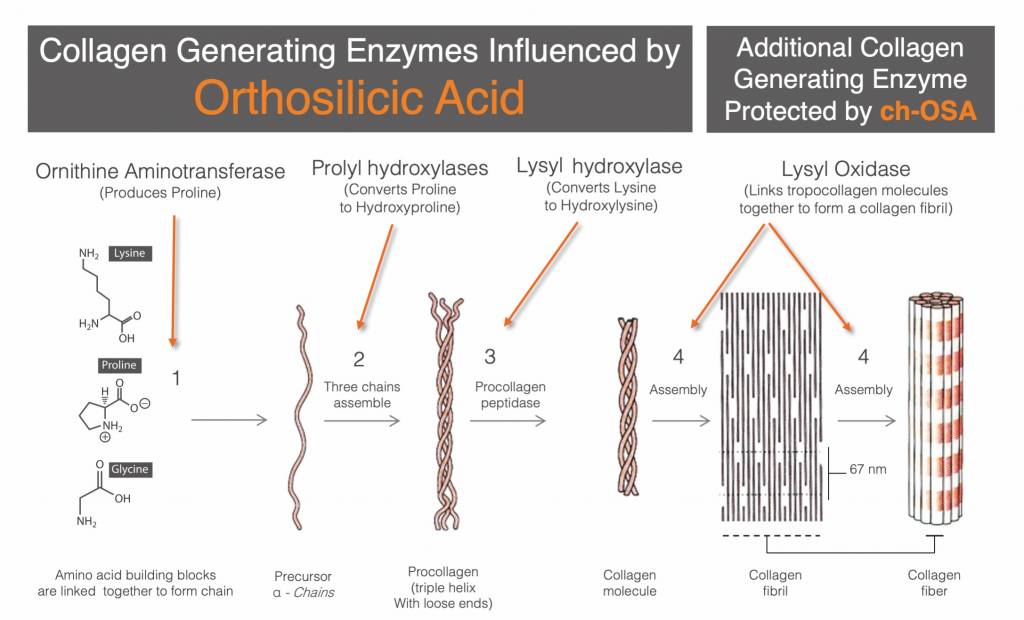
Procollagen peptidase enzymes are another example. They cut the “loose ends” of triple helix structures to form a tropocollagen molecule—or a single collagen molecule.
Afterwards, lysyl oxidase enzymes link tropocollagen molecules together to form fibrils and then link fibrils together to form collagen fibers.
Collagen generation is clearly a multi-step process. Some steps occur with collagen-generating fibroblasts while others occur extracellular. More than 30 genes are involved in regulating the process and at least 10 enzymes must be activated to complete the job.
The series of mostly enzymatic driven post-translational modifications converting the alpha chains into collagen is called the “procollagen cycle.” And if a developing collagen molecule doesn’t progress through the cycle at an adequate pace, the body seems to give up on developing that particular molecule and tears down that slowly developing collagen molecule into the basic amino acids and starts the whole process again (2).
The same group of researchers estimating that the body must make 100 grams/day of collagen to maintain 7 key tissues, actually estimate the body must make about 924 grams of procollagen to successfully yield that 100 grams of mature collagen.
| Organ | Estimated Collagen Content (grams) | Net Collagen Synthesis (g/day) | Maximal Procollagen Cycle Rate | Gross Procollagen Synthesis (g/day) |
| Skeleton | 1,640 | 43.3 | 90% | 433 |
| Skin | 1,390 | 27.8 | 57% | 64 |
| Muscle | 523 | 18.4 | 95% | 392 |
| Cartilage | 109 | 4.5 | 75% | 18 |
| Heart | 3.36 | 0.3 | 96% | 9 |
| Lung | 18.4 | 1.3 | 82% | 7 |
| Ligaments | 45 | 0.8 | 20% | 1 |
| Total | 3,729 | 97 | 924 |
The collagen generation construction process may sound cumbersome and inefficient. And it can be, especially if someone isn’t properly nourished.
In my opinion, it’s quite clear: The lower the amount of the required enzyme-activating nutrients someone has, the less efficient the procollagen cycle will be and less collagen will be produced.
RAP:In my opinion, for most people, the limiting nutrient will be silicon. Please tell us more about silicon’s roles in this process.
RPJr: Enzymes are powerful factory workers in the body, but enzymes require nutrients as co-factors to be activated and to do their job. Many of silicon’s roles are related to activating enzymes.
There is some discussion whether silicon is involved in making mRNA. But this is still rather speculative and a topic of spirited discussion at times. Time and additional research will tell if silicon’s first role in supporting collagen production is by increasing mRNA production.
Silicon, as orthosilicic acid (OSA), definitely is involved with proline production. Proline is the second most abundant amino acid in procollagen and collagen. One of the ways the body makes proline is by using the enzyme, ornithine aminotransferase, to convert ornithine into proline. Ornithine aminotransferase requires OSA to function (3).
Prolyl hydroxylase enzymes are also OSA dependent (4). Without adequate silicon intake, the body can’t convert proline into hydroxyproline, a critical component in making collagen triple helix structures.
It’s important to note the body must make hydroxyproline during the collagen production process. The body needs to make its own hydroxyproline for use in collagen. The body can’t put dietary hydroxyproline directly into the alpha chain. There’s no codon or DNA triplet code for hydroxyproline.
In addition to ornithine aminotransferase, and prolyl hydroxylase enzymes, lysyl hydroxylase is also orthosilicic acid dependent (5). That’s three sets of enzymes requiring orthosilicic acid.
In addition, some orthosilicic acid can be used to link collagen fibers together with one another or link them to proteoglycans and/or glycosaminoglycans to strengthen the overall collagen network.
Furthermore, there is some discussion as to whether lysyl oxidase is OSA-dependent or somehow positively influenced by OSA. This is also an area to keep an eye on in coming years as research evolves.
Bio Minerals’ product, BioSil, contains choline-stabilized OSA. BioSil improves collagen production in some additional ways as well.
As an example, glycine is the most abundant amino acid in collagen. There are some estimates that the body requires as much as 12 grams of glycine a day to produce an adequate amount of collagen. It’s a good thing the body can make some of its own and is pretty good at recycling it. BioSil can help the body make its own glycine. The glycine hydroxy methyltransferase pathway the body uses to convert serine into glycine requires choline– an essential B vitamin-like compound or it’s metabolite betaine (2). BioSil is a great source of choline, a nutrient that perhaps as much as 80% of the U.S. population doesn’t obtain the minimum RDA or the Institutes of Medicine’s Adequate Intake (AI) in their diet (6).
Also, BioSil helps protect lysyl oxidase from being damaged by homocysteine by helping to convert the damaging homocysteine into beneficial methionine. If left unchecked, homocysteine interferes with and suppresses collagen production by interfering with lysyl oxidase activity (7).
RAP: What about elastin production?
RPJr:Elastin is also produced by fibroblasts using many of the same enzymes. As an example, collagen and elastin are the only two proteins containing hydroxyproline. So, the same ornithine aminotransferase and prolyl hydroxylase enzymes required to build collagen are also required to build elastin (8).
In addition, lysyl oxidase is required to link together tropoelastin molecules into fibrils and fibers the same way it links tropocollagen molecules into fibrils and fibers.
Another important similarity is that glycine is also the most predominant amino acid in Elastin.
RAP: Skin is so important, but it is constantly being damaged by everything from sunlight to physical injury. Yet, its repair process is extremely complicated as you have just described with the formation of collagen and elastin. What about nails and hair? That's an interesting story for another day.
References
- Miyahara T, Shiozawa S, Murai A. The Effect of Age on Amino Acid Composition of Human Skin Collagen. Journal of Gerontology. 1978. Vol. 33. No. 4. 498-503
- Meléndez-Hevia E, De Paz-Lugo P, Cornish-Bowden A and Cárdenas M L 2009 A weak link in metabolism: the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis; J. Biosci. 34 853–872] DOI 10.1007/s12038-009-0100-9
- Seaborn and Nielsen: Silicon deprivation decreases collagen formation in wounds and bone, and ornithine transaminase enzyme activity in the liver. Biol Trace Elem Res 56:153-165
- Calomme MR, Vanden Berghe DA (1997). Supplementation of calves with stabilized orthosilicic acid. Effect on the Si, Ca, Mg and P concentrations in serum and the collagen concentration in skin and cartilage. Biol Trace Elem Res, 56:153-165.
- Kim et al. Effect of Vitamin C, Silicon and Iron on Collagen Synthesis and Break-Down Enzyme Expression in the Human Dermal Fibroblast Cell (HS27). Korean J Nutrition. 2009; 42(6): 505-515
- RDA Monograph Reference
- Thaler R et al. (2011) Homocysteine suppresses the expression of the cross-linker lysyl oxidase. J Biol Chem, 286, 5578-5588.
- Foster JA, Bruenger E, Gray W, Sandberg L. Isolation and Amino Acid Sequences of Tropoelastin Peptides. The Journal of Biological Chemistry. Vol. 248, No. 8, Issues of April 25, pp. 2876-2879, 1073.










