Last month, we began our discussion about magnesium being one of the most important nutrients with Andrea Rosanoff, Ph.D. We examined how magnesium is involved in nearly every chemical reaction in the body that requires energy to proceed. Paramount is magnesium’s role in activating adenosine 5’-triphosphate (ATP)—a biochemical that Dr. Rosanoff called the “Battery of Life.”
Whenever any reaction of life requires energy, ATP is most likely necessary for that reaction to proceed, and most ATP is actually in the form of a chemical complex with magnesium (Mg-ATP) within the cells. In addition, magnesium is directly involved in hundreds of reactions including both those reactions driven by having the magnesium bind to the substrate (the chemical being acted upon) and those driven by magnesium binding to the enzyme. Magnesium itself is a direct co-factor for over 600 enzymatic reactions involving DNA and RNA synthesis, protein synthesis, glucose uptake and metabolism, just to name a few highlights (1). Therefore, few chemical reactions can occur in the body without magnesium and important reactions will proceed for optimal health only when adequate magnesium is available.
Magnesium’s litany of actions goes on and on; the mineral is implicated in several physiological processes such as vitamin D biochemistry, hormone synthesis and secretion processes, nerve cell function, digestion and muscle contraction/relaxation. It is part of cell membrane structure including membranes of the cell nucleus, mitochondria and the endoplasmic reticulum, which gives cells their internal structure.
If anyone needs more reason to respect magnesium, its role in activating vitamin D should do the job. Vitamin D metabolism involves at least eight steps dependent on magnesium. After decades of essentially ignoring the importance of vitamin D, we have now come to learn how important vitamin D is. That has become clear after learning that vitamin D is required to activate more than 2,000 genes. We discussed this with Dr. John H. White in our March 2013 column.
The need for magnesium to activate vitamin D is the main point that Dr. Rosanoff will discuss with us this month. We will also talk about the role of magnesium as an electrolyte. In future columns, we will cover the role of magnesium in specific health conditions including mental and nerve function, heart disease, diabetes and other chronic conditions.

|
|
| Andrea Rosanoff, Ph.D. |
Dr. Rosanoff is a nutritional biologist and the director of research at The Center for Magnesium Education & Research, LLC in Pahoa, HI. Dr. Rosanoff spent her undergraduate years at UC Berkeley studying biological sciences and then taught science for several years before returning to graduate school. After earning her M.S. and Ph.D. degrees in nutrition from the University of California at Berkeley in 1982, Dr. Rosanoff began her study of nutritional magnesium, especially as it relates to health in the developed world. Her work as senior chemical specialist for Dialog Information Services and Information Analyst at Chevron Res & Tech. Corp. gave her early training of and access to the online scientific literature, which she now uses in her research and teaching on all aspects of nutritional magnesium.
Her main research interests include development of the nutritional magnesium paradigm of cardiovascular disease, the role of magnesium nutrition in osteoporosis, diabetes, psychobiological and renal disease, global spreading and generational effect on nutritional magnesium of the processed food diet, and the role of magnesium in stress reactions. Some of her recent publications include studies on changing food crop magnesium concentrations and their possible impact on human health, magnesium supplementation and hypertension, a comparison of magnesium supplements with statin pharmaceuticals, and the rising calcium to magnesium ratio of dietary intakes in the United States.
 In addition to her work presented in peer-reviewed journals and at scientific conferences, Dr. Rosanoff is interested in explaining health aspects of nutritional magnesium to the general public. She co-authored with the late M.S. Seelig, M.D. a book entitled, The Magnesium Factor (published in 2003), which discusses scientific literature through 2002 on nutritional magnesium’s impact on risk factors for cardiovascular disease. She wrote, produced and narrated an animated two-minute video entitled, “Balancing Calcium and Magnesium” in 2008, which can be viewed at her Web site (www.MagnesiumEducation.com). Through the Center for Magnesium Education & Research, Dr. Rosanoff continues to foster knowledge of magnesium research; with IAPN the Center sponsors International Symposia bringing together magnesium researchers from all over the world to discuss and present research on magensium in agriculture and its connection to human nutrition. The Center is also spearheading a group to apply to the U.S. Food and Drug Administration for a health claim for magnesium in hypertension plus a second effort to reevaluate the serum magnesium reference range.
In addition to her work presented in peer-reviewed journals and at scientific conferences, Dr. Rosanoff is interested in explaining health aspects of nutritional magnesium to the general public. She co-authored with the late M.S. Seelig, M.D. a book entitled, The Magnesium Factor (published in 2003), which discusses scientific literature through 2002 on nutritional magnesium’s impact on risk factors for cardiovascular disease. She wrote, produced and narrated an animated two-minute video entitled, “Balancing Calcium and Magnesium” in 2008, which can be viewed at her Web site (www.MagnesiumEducation.com). Through the Center for Magnesium Education & Research, Dr. Rosanoff continues to foster knowledge of magnesium research; with IAPN the Center sponsors International Symposia bringing together magnesium researchers from all over the world to discuss and present research on magensium in agriculture and its connection to human nutrition. The Center is also spearheading a group to apply to the U.S. Food and Drug Administration for a health claim for magnesium in hypertension plus a second effort to reevaluate the serum magnesium reference range.
Passwater: Dr. Rosanoff, the importance of vitamin D is becoming widely appreciated, but few people realize that magnesium is so critical to the activation of vitamin D. I understand that you are co-authoring a review article on magnesium and vitamin D. Is that correct?
Rosanoff: Yes. I am working on a review of this subject with Professor Qi Dai of Vanderbilt University, who was the senior author of the team that recently published a very interesting article about a possible connection between vitamin D and nutritional magnesium status and common chronic diseases, and Professor Sue Shapses of Rutgers University, who is an expert in vitamin D, calcium and osteoporosis.
Passwater: Magnesium is needed for the formation of vitamin D in our skin from its precursors, a process initiated by sunlight. How is magnesium involved here?
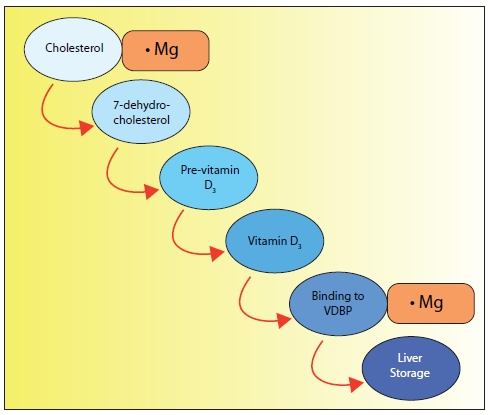
Rosanoff: First of all, the precursor to vitamin D synthesis in the skin is cholesterol, and magnesium is an important co-factor for the rate-limiting enzyme for cholesterol production. (When cellular magnesium is low, cholesterol production is not well controlled and can be overproduced in the cell.) Beyond that, magnesium is critical for the synthesis of vitamin D in this skin reaction set in play by sunlight. In addition, the vitamin D3 so synthesized is bound to a protein for transport, and magnesium is necessary for this binding. And of course, magnesium is necessary at several stages of protein synthesis.
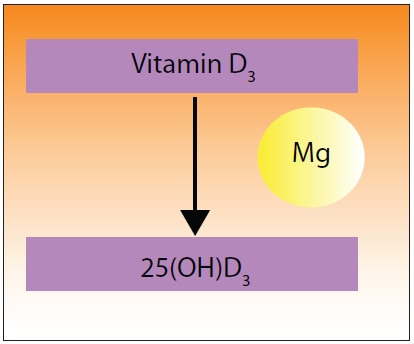
Figure 1 shows the major steps involved.
|
| Figure 1: Magnesium is required in many steps along the way to making vitamin D useful to the body. In Figure 1, we note that magnesium is important in cholesterol regulation. Cholesterol is involved in the first step in making vitamin D in the skin. Magnesium is also required for the binding of vitamin D to its carrier, Vitamin D Binding Protein (VDBP), which transports the newly formed vitamin D to the liver where it can be converted to 25-hydroxyvitamin D3, a required step along its pathway to the active form of vitamin D3. |
|
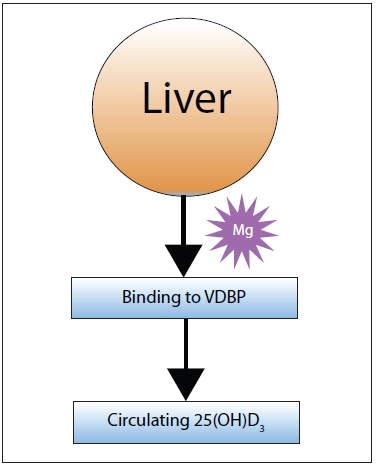
Figure 2: Magnesium is also required in the step that activates vitamin D to 25-hydroxy |
|
| Figure 3: Full vitamin D activation doesn’t occur until 25-hydroxyvitamin D3 (25[OH]D3) reaches the kidneys, which requires binding to the Vitamin D Binding Protein (VDBP). |
Passwater: Vitamin D is actually a hormone once it is activated in the liver and kidneys. Please tell us how magnesium is involved in activation by the liver.
Rosanoff: Vitamin D3, produced in the skin, is not an active form. In the liver, it is “hydroxylated” to 25-hydroxyvitamin D3 (25-OH D3). This step is also magnesium dependent.
Passwater: After being converted to 25-hydroxyvitamin D3, further action is required in the kidneys, so it needs to leave the liver to get to the kidneys.
Rosanoff: Yes, and again, magnesium is needed to help carry the 25-hydroxyvitamin D3 out of the liver.
Passwater: Please explain the role of magnesium in the kidney’s activation of vitamin D. What is going on here?
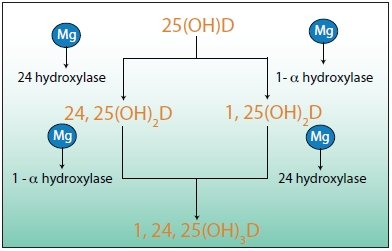
Rosanoff: The most active form of vitamin D in terms of calcium absorption is the form called 1,25 di-hydroxyvitamin D3, which is formed in the kidney by “hydroxylating” the 25-hydroxyvitamin D3 synthesized in the liver and circulating in the bloodstream connected to the vitamin D binding protein. There are a couple of pathways in the kidney metabolism that accomplish this “hydroxylation,” but all reactions appear to require magnesium (See Figure 4).
|
|
| Figure 4: In the kidney, 25-hydroxyvitamin D3 is converted to the fully active vitamin/hormone 1,25-dihydroxy-vitamin D3 and closely related forms. This conversion is facilitated by hydroxylase enzymes, which require magnesium. Thus, magnesium is involved in every step of the process of activating vitamin D. |
As I mentioned before, 1,25 di-hydroxyvitamin D3 has high activity in stimulating calcium absorption in the intestine. This is an important part of calcium regulation and control in the body. Also important is para-thyroid hormone or PTH. When circulating calcium gets too low, PTH synthesis and secretion is stimulated, and magnesium is necessary for these synthesis and secretion processes. PTH not only stimulates the hydroxylation of 25-hydroxy-D3 in the kidney to its more active form, 1,25 di-hydroxy D3 thus enriching intestinal absorption of calcium, it also stimulates the release of calcium from the bone. These actions of PTH help keep the calcium level in the blood from going too low. However, when magnesium status is so low that one experiences a low blood magnesium level, proper calcium levels of the blood cannot be attained, even with all the active D3 and PTH at work. In such cases, magnesium status must be brought up in order for proper calcium serum levels to be achieved.
With so many places in the vitamin D story where magnesium is essential, one might expect circulating levels of 25-hydroxy D to be affected by low magnesium status. And, this has been shown recently: groups of people with high intakes of magnesium (both food and supplements) show reduced risk of vitamin D deficiency and/or insufficiency as measured by serum 25-hydroxy D. As your readers may know, there has been much information recently about vitamin D status, and many are taking vitamin D supplements since higher levels of serum 25-hydroxy D have been associated with lower mortality due to cardiovascular disease and colorectal cancer as well as other health issues. If a person is unknowingly low in their magnesium status and has a serum vitamin D test, it is quite possible that the serum vitamin D level will register low as a result of the low magnesium status, and low magnesium status has been shown to be associated with higher risks of cardiovascular disease, colorectal cancer and other issues now currently associated with low vitamin D status.
 In other words, it seems possible that a low magnesium status might be causing both risks for these diseases as well as the low serum vitamin D. Is this a problem? Is it okay to take high amounts of vitamin D when magnesium status is low? I would say that it is always best to have adequate amounts of all essential nutrients for optimal health. But in the case of vitamin D, I would suggest that a person ascertain their magnesium status and make sure it is adequate before embarking on a high oral vitamin D therapy.
In other words, it seems possible that a low magnesium status might be causing both risks for these diseases as well as the low serum vitamin D. Is this a problem? Is it okay to take high amounts of vitamin D when magnesium status is low? I would say that it is always best to have adequate amounts of all essential nutrients for optimal health. But in the case of vitamin D, I would suggest that a person ascertain their magnesium status and make sure it is adequate before embarking on a high oral vitamin D therapy.
Extra vitamin D enabling more calcium absorption without adequate magnesium is not the optimal way to achieve preventive health. Extra calcium in the face of inadequate magnesium can bring on issues of calcification of soft tissues and over-action of cells in the “fight or flight” reaction (See Resnick articles of the 1990s and the two-minute video on my Web site www.MagnesiumEducation.com). Balance of calcium with magnesium is an important aspect of long-term health, and it is especially important in these days where the calcium-to-magnesium ratio has been rising in the United States steadily since 1977. Traditionally, a two-to-one calcium-to-magnesium intake ratio has been recommended as a maximum (i.e., you can go lower than this but not higher). And, today in the United States, that ratio is above three and in some groups such as older women who are taking calcium supplements, the ratio is above four. This is not a healthy calcium-to-magnesium ratio, and adding vitamin D supplements in the face of such high ratios can only exacerbate the negative effects. It is better to bring one’s magnesium status up, recheck the serum vitamin D level and then go from there.
Passwater: The energy-producing and vitamin D-activating functions of magnesium are amazing, but there are still other important roles. Let’s chat about magnesium’s role as an electrolyte. Let’s start at the beginning. What are electrolytes?
Rosanoff: Electrolytes are positively charged metal ions that are active in biological systems. The most common and most prevalent are calcium (Ca+2), magnesium (Mg+2), sodium (Na+1) and potassium (K+1). The calcium and magnesium ions each have a +2 charge, and the sodium and potassium ions each have a +1 charge. For the most part, magnesium and potassium ions are held within the cell and sodium and calcium ions are outside the cell membrane. There is an important balance between calcium and magnesium and there is an important balance between sodium and potassium. At this time in the United States, just about everyone is getting too much sodium in their diets as well as too little potassium in their diets. Low potassium has been implicated in heart health and stroke—nurses getting above 3,000 mg/day potassium showed no strokes in one study, for example. The emphasis has been on lowering sodium intake, which is difficult to do as much of it is put into our foods during processing, but what is often overlooked is how low our intakes of potassium are. Fruits and vegetables are the good sources of potassium, so you can see that “eating your vegetables” is how to best balance one’s sodium intake with a good potassium intake.
In addition to potassium nutrition being at the low end of healthy intake while sodium intake is quite high, magnesium intake in the United States is also low while in some groups, calcium can be quite  high, especially when people take calcium supplements. And, as I’ve mentioned previously, the calcium intake from foods in the United States has been rising steadily for many years while the intake of magnesium has remained pretty much steady.
high, especially when people take calcium supplements. And, as I’ve mentioned previously, the calcium intake from foods in the United States has been rising steadily for many years while the intake of magnesium has remained pretty much steady.
Passwater: It’s amazing how much magnesium is involved in producing energy and activating vitamin D. Does this make an actual difference in longevity? Let’s discuss that in the next part of this series. WF
Dr. Richard Passwater is the author of more than 45 books and 500 articles on nutrition. Dr. Passwater has been WholeFoods Magazine’s science editor and author of this column since 1984. More information is available on his Web site, www.drpasswater.com.
Reference
1. J.H. de Baaij, J.G. Hoenderop and R.J. Bindels, “Magnesium in Man: Implications for Health and Disease,” Physiol. Rev. Physiol. Rev. 95 (1), 1-46 (2015).
This magnesium series is sponsored by Natural Vitality.
Published in WholeFoods Magazine, March 2015