Last month, Jørn Dyerberg, M.D., Dr. Med. Sc., and I discussed the false report that fish oil intake was shown to be a risk factor in prostate cancer. This month, I will continue discussing this issue with longtime omega-3 expert and co-inventor of the HS-Omega-3 Index test, William Harris, Ph.D. Dr. Harris presents several reasons why we should ignore the inappropriate report and continue eating a diet rich in fatty fish and consuming supplements rich in EPA and DHA for their many health benefits.
Dr. Harris is one of the world’s leading omega-3 researchers. He holds a Ph.D. in nutritional biochemistry from the University of Minnesota and began his omega-3 research in 1979 as a post-doctoral fellow in the laboratory of William Connor, M.D., at the Oregon Health Services University (Portland). Dr. Connor was a leading researcher at the time in nutrition, lipids (fats) and heart disease. Dr. Harris’ early research centered on the possible effects of large quantities of salmon oil on blood cholesterol.
Dr. Harris was the director of the Lipid Research Laboratories at the University of Kansas Medical Center (KUMC) and of the Mid America Heart Institute, both in Kansas City, MO, for 22 years, and was on the faculty at KUMC and at the University of Missouri-Kansas City School of Medicine. Between 2006 and 2011, he was the director of the Cardiovascular Health Research Center at Sanford Research/USD (Sioux Falls, SD).
Over the last 32 years, Dr. Harris’ research has focused primarily on omega-3 fatty acids and cardiovascular disease. He has been the principal investigator on five omega–3-related NIH grants, and is currently evaluating omega-3 blood tests as a possible new risk factor for cardiovascular disease. In 2004, he and his colleague Clemens von Schacky proposed that the Omega-3 Index be considered as a new cardiovascular risk factor, and in 2009, he founded OmegaQuant, LLC to offer the test commercially. In 2011, he became a senior scientist at Health Diagnostic Laboratory, Inc. in Richmond, VA. He retains an academic appointment as professor of medicine at the Sanford School of Medicine, University of South Dakota in Sioux Falls.
Over the years, Dr. Harris has published many major research articles and reviews on the marine lipids, EPA and DHA. A major discovery of Dr. Harris and his colleagues has been the elucidation of the Omega-3 Index as a risk factor in cardiovascular disease. Dr. Harris was kind enough to discuss the Omega-3 Index with us in October 2011.
Dr. Harris’ leadership role in omega-3 research was the reason that Dr. Dyerberg (the discoverer of fish oil’s role in reducing cardiovascular risk) and I asked Dr. Harris to write the foreword to our recent book, The Missing Wellness Factors: EPA and DHA (1).
Passwater: Dr. Harris, before we discuss the unfounded reports circulating in the media about fish oil being linked to prostate cancer, let’s start with some basics about measuring the primary beneficial fats found in fish oil, the omega-3s EPA and DHA. Let’s begin with the HS-Omega-3 Index.
Harris: The “HS-Omega-3 Index” is the proprietary test that is commercially available for physicians and researchers (2). The “HS” refers to Harris and von Schacky. The HS-Omega-3 Index measures the fatty acid content of red blood cells (RBC). Figure 1 depicts an RBC with a section of its lipid bilayer expanded to show membrane phospholipids (a circle with two squiggly lines attached constitutes one phospholipid molecule). Each of the 32 molecules shown here has two fatty acids (FA); those in red are the long-chain omega-3 FA EPA and/or DHA. With a total of 64 FA, three long-chain omega-3 FA constitute 4.6% of the total FA. Hence, the Omega-3 Index of the patient from whom this cell was taken would be 4.6%.
Passwater: What are the advantages of using RBC for the measurement of EPA and DHA over simply measuring the plasma levels?
Harris: RBC omega-3 levels are 1) more biologically stable (i.e., they hardly vary at all within each person from week to week), 2) technically easier to analyze (especially compared with plasma phospholipids), 3) more reflective of long-term omega-3 intake, 4) well-studied and linked with risk for major diseases, 5) well-correlated with most other metrics of omega-3 status and 6) less influenced by sporadic fish/fish oil intake.
Passwater: In looking at the Brasky et al., publication, I note that they did not use the HS-Omega-3 Index, but used plasma phospholipids to measure the omega-3 levels (3). Since plasma omega-3 levels are a less reliable measure of long-term omega-3 status compared to RBC levels, wouldn’t the results reflect more recent diet rather than long-term diet?
Harris: Plasma phospholipid omega-3 level 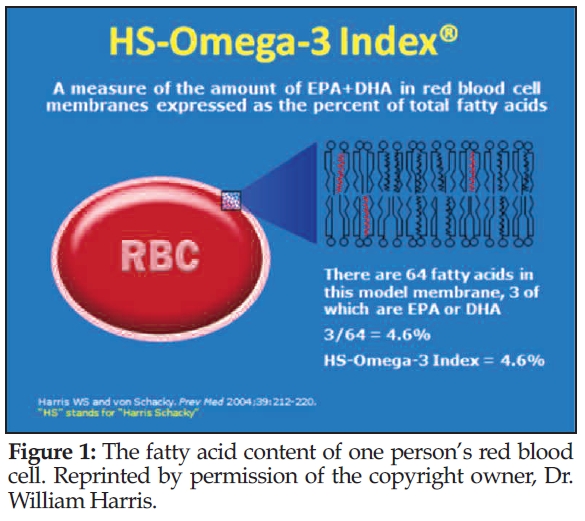 measurements are more “noisy” (i.e., vary day to day) than RBC omega-3 levels, and they are more easily perturbed by a recent fish meal or taking fish oil pills. But in reality, people eat fatty fish very rarely in this country, and the vast majority do not (and certainly did not 10 years ago when the prostate study started) take fish oil supplements. So, I think the plasma phospholipid omega-3 levels they measured were OK, particularly since they were all averaged together. What happens is that today, Joe’s plasma omega-3 is a bit higher than usual, but Sue’s level is a bit lower than usual…so on a population basis, it averages out.
measurements are more “noisy” (i.e., vary day to day) than RBC omega-3 levels, and they are more easily perturbed by a recent fish meal or taking fish oil pills. But in reality, people eat fatty fish very rarely in this country, and the vast majority do not (and certainly did not 10 years ago when the prostate study started) take fish oil supplements. So, I think the plasma phospholipid omega-3 levels they measured were OK, particularly since they were all averaged together. What happens is that today, Joe’s plasma omega-3 is a bit higher than usual, but Sue’s level is a bit lower than usual…so on a population basis, it averages out.
In population research studies in which hundreds of people’s levels are being measured and averaged together, plasma phospholipids are fine and, in fact, have been used in many studies. Typically, higher levels of plasma phospholipid omega-3 are associated with better health outcomes down the road. I prefer RBCs and they are definitely better in patient care settings where the doctor takes a single blood test and assumes that the values s/he gets reflect the patient’s “normal” status.
This is analogous to measuring “hemoglobin A1c” (or glycated hemoglobin) instead of plasma glucose to monitor blood sugar in diabetic patients. The former is an RBC-based test and reflects what doctors really want to know (i.e., the average glucose levels in the patient over weeks). The results of the RBC-based test are not influenced by eating a meal like is the case with the plasma glucose level. Similarly, eating a big meal of salmon the night before or taking a lot of fish oil in pills on the morning of a blood test can artificially elevate plasma omega-3 levels and misrepresent long-term omega-3 status. The effect takes about two days to wash out. That’s why RBCs are better, but plasma levels are not bad.
The most common reason for not using RBCs to measure omega-3 levels in research studies is the simple fact that in many studies that started years (or decades) ago, nobody thought about saving RBCs; they were simply discarded after the plasma was removed and frozen.
Passwater: So, you don’t consider the omega-3 measurement used by Brasky et al., a serious flaw?
Harris: No, I do not. Again, exactly the same test has been used in other studies with very favorable outcomes. I can’t criticize the use of plasma tests in studies for which I don’t like the outcome and embrace it in studies that are favorable. That’s being inconsistent and biased.
 My main criticism of the Brasky report is not so much that the study was flawed as that it was completely misinterpreted and overspun. Their conclusions reached far beyond their actual findings. The measurement they used was plasma phospholipid omega-3.
My main criticism of the Brasky report is not so much that the study was flawed as that it was completely misinterpreted and overspun. Their conclusions reached far beyond their actual findings. The measurement they used was plasma phospholipid omega-3.
Another important study that used the same test found that a higher omega-3 level was associated with increased longevity (i.e., lower risk for death) by over two years in a large group of older Americans (4). Another study using the same marker showed that people with higher (compared with lower) plasma phospholipid omega-3 levels were less likely to develop atrial fibrillation (common heart rhythm problem). So, if we reject the use of plasma phospholipid omega-3 levels in one unfavorable study, then how can we accept it in other studies that have results we agree with? Again, the problem here was not with the use of plasma phospholipid fatty acid measurements; it was in the misinterpretation of the findings.
Passwater: How about other omega-3 studies and prostate cancer?
Harris: A study from the Harvard group published in 2007 found that higher levels of omega-3 fatty acids in whole blood were related to reduced risk of developing prostate cancer over a 13-year period (5). This is exactly the opposite of what Brasky reported. Why did this paper not get the press that Brasky got? There have been several prostate cancer studies using a variety of markers of omega-3 status, and most of them have been neutral, meaning no statistically significant relationship with risk. This perspective was missing from the Brasky paper.
Passwater: What studies have been done using the HS-Omega-3 Index and what was found?
Harris: We found many things, such as:
1. the Omega-3 Index was only partly determined by omega-3 intake and that person-to-person differences were not even 50% explained by differences in intake;
2. an Omega-3 Index of greater than 8% was associated with a lower risk for acute coronary syndromes (ACS). ACS is an umbrella term used to cover any group of clinical symptoms compatible with acute myocardial ischemia. Acute myocardial ischemia is chest pain due to insufficient blood supply to the heart muscle that results from coronary artery disease (also called coronary heart disease);
3. a suite of 10 RBC fatty acids (including omega-3, omega-6, saturated and trans-fatty acids) could discriminate between ACS patients and controls better than the standard risk factors (age, sex, smoking, cholesterol, HDL, blood pressure and diabetes);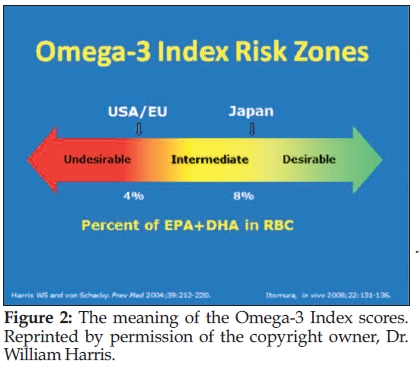
4. a higher Omega-3 Index was associated with slowed “cellular aging” as measured by the five-year rate of telomere shortening;
5. heart patients with below average Omega-3 Index values died at a faster rate than those with above average levels;
6. patients with severe sleep apnea have lower RBC DHA levels than people who have less severe disease;
7. patients admitted to the hospital with an acute myocardial infarction are less likely to develop ventricular arrhythmias if they have a higher versus a lower Omega-3 Index;
8. inflammatory markers were inversely related to the Omega-3 Index;
9. older people seem to have higher Omega-3 Index values than younger people;
10. a lower Omega-3 Index was associated with depression, both in adolescent patients and in post ACS patients;
11. a slower heart rate was related to a higher Omega-3 Index; and
12. the Omega-3 Index was directly associated with cardiac exercise parameters in patients with stable coronary heart disease (CHD).
Passwater: In terms of heart disease, what are good and bad scores with the HS-Omega-3 Index?
Harris: Good is above 8% and bad is below 4% (see Figure 2).
Passwater: Since the recent study has caused many people to question whether they should reduce their fish intake and/or give up fish oil supplements, people need to know that the so-called study did not prove anything and is based on poor “science.” Dr. Dyerberg and I have discussed some of the flaws in the report, but what flaws do you see?
Harris: My concerns fall into the following eight categories:
1. Very low levels of omega-3 were observed in everybody, not just those who went on to develop prostate cancer. They were 3.62% in the “no-cancer” control group and 3.74% in the “high-grade cancer” group. This difference is very small and would have no meaning clinically as they are within the normal lab variation.
Based on experiments in our lab, the lowest quartile (25%) of values would correspond to an HS-Omega-3 Index of less than 3.16% and the highest to an Index of greater than 4.77%. These values are obviously low, and virtually none of the subjects were in “danger” of having an HS-Omega-3 Index of above 8%. In Framingham, the mean Omega-3 Index of participants who were not taking fish oil supplements was 5.2% and for those taking supplements, it was 7.5%. Both of these numbers are considerably higher than the values reported by Braskey et al., even in their highest quartile.
2. Fish oil supplements were not part of this study. Based on the omega-3 levels above, virtually nobody was taking supplements; and the investigators did not record fish intake, so we don’t know how much fish (especially oily fish) they were eating.
3. In one of many interviews given by the senior author of the Brasky study (Alan Kristal, DrPH), in response to the question of whether older men should stop taking fish oil supplements, he said, “Yes. There is no benefit, and now we have identified harm.” THIS is the biggest problem with the study in my view: the authors are wildly and irresponsibly extrapolating beyond their own data and drawing conclusions that their study did not even address. One senses a pre-existing bias against supplements in statements like this.
4. But even granting that the associations between omega-3 levels and subsequent development of prostate cancer are real, the authors cannot conclude that the higher omega-3 levels CAUSED the cancer. Associations do not indicate causation. (This is a basic “Logic 101” error that well-trained scientists should never make.)
There are some studies that have shown that cancer cells can actually cause an increased synthesis of EPA and DHA, so it’s conceivable that it was the pre-clinical, slow-growing cancer cells that caused the (very small) increases in omega-3, not the reverse. But even without that, simply finding that biomarker X is higher in people that develop disease Y proves nothing whatever; it is what we call a “hypothesis generating” result, essentially a suggestion that further research should focus on this possible (but unproven) connection.
5. It is important to put these findings into perspective (which the authors failed to do). First, consider the risk of dying from prostate cancer versus ischemic heart disease (IHD). Based on the National Vital Statistics Report for deaths in U.S. males in 2010, there were about 28,500 deaths from prostate cancer and 207,500 deaths from IHD; that’s a 7.3 times higher rate of death from heart disease (6). If one makes the rather extreme assumptions that higher omega-3 intake reduces the risk of death from heart disease by only 10% and increases the risk of death from prostate cancer by 50%, then the chances of dying from CHD are still more than four times greater than dying from prostate cancer. This very crude analysis suggests that even in the worst-case scenario, the benefit of higher omega-3 intakes/levels still outweighs the risk.
6. The authors also failed to present the fuller story taught by the literature. The same team reported in 2010 that the use of fish oil supplements was not associated with any increased risk for prostate cancer (7). A 2010 meta-analysis of fish consumption and prostate cancer reported a reduction in late-stage or fatal cancer among cohort studies, but no overall relationship between prostate cancer and fish intake (8). Terry et al. in 2001 reported higher fish intake was associated with lower risk for prostate cancer incidence and death, and Leitzmann et al. in 2004 reported similar findings (9, 10).
Higher intakes of canned, preserved fish were reported to be associated with reduced risk for prostate cancer. Epstein et al. found that a higher omega-3 fatty acid intake predicted better survival for men who already had prostate cancer, and increased fish intake was associated with a 63% reduction in risk for aggressive prostate cancer in a case-control study by Fradet et al. (11,12). So, there is considerable evidence actually FAVORING an increase in fish intake for prostate cancer risk reduction.
7. Another relevant question to ask is how prostate cancer rates in Japan (where fish intake is high and the HS-Omega-3 index averages more than 9%) compare to those in the United States (where the average Index is 4–5%). Here is a quote from the World Foundation of Urology:
“Prostate cancer incidence is really high in North America and Northern Europe (e.g., 63 per 100,000 white men and 102 per 100,000 Afro-Americans in the United States), but very low in Asia (e.g., 10 per 100,000 men in Japan)” (13).
So, in a setting of high fish intake and high blood omega-3 levels, one finds very low rates of prostate cancer. Hmmmm…
8. There is also a wealth of evidence from randomized clinical trials with fish oils in which the endpoint of interest is usually cardiovascular disease. However, in each study, the incidence of cancer is tracked as well. The findings for eight major cardiovascular studies reported to date included over 78,000 patients. In none of these studies was cancer incidence significantly increased by omega-3 fatty acid supplementation.
In summary, although the work of Brasky et al. does add to the evidence base for omega-3 fatty acids and prostate cancer, it is far from definitive. Had this been a study in which men were randomly assigned to fish oil supplements or placebo, and then the incidence of prostate cancer was followed over the years, and cancer rates were higher in the fish oil than the placebo group, THEN there would be grounds for the kinds of claims the authors made. But when it’s nothing but tiny differences in omega-3 blood levels being associated with slightly more cancer risk over nine years, nothing can legitimately be said about what caused what. It is completely inappropriate to condemn fish oil supplements or eating fish based on this study. There will always be mixed findings in studies of “diet” and “disease” since both predictor (diet or diet-related biomarkers) and outcome (disease) entail so many variables, known and unknown. Nevertheless, the risk/benefit ratio for fish and fish oils remains very favorable.
Passwater: What is your advice to our readers regarding the Brasky  report?
report?
Harris: Ignore it. Continue to eat fish like salmon, sardines, herring and albacore tuna, and keep taking (as I do) fish oil supplements.
Passwater: Dr. Harris, thank you once again for discussing omega-3s and health with our readers. WF
Dr. Richard Passwater is the author of more than 45 books and 500 articles on nutrition. Dr. Passwater has been WholeFoods Magazine’s science editor and author of this column since 1984. More information is available on his Web site, www.drpasswater.com.
References
1. J. Dyerberg and R.A. Passwater, The Missing Wellness Factors—EPA and DHA (Basic Health Publications, Inc., Laguna Beach, CA, 2012).
2. W.D. Harris and C. von Schacky, “The Omega-3 Index: A New Risk Factor for Death from CHD?” Preventive Med. 39, 212–220, (2004).
3. T.M. Brasky, et al., “Plasma Phospholipid Fatty Acids and Prostate Cancer Risk in the SELECT Trial,” J. Natl. Cancer Inst. (2013); doi: 10.1093/jnci/djt174; First published online: July 10, 2013.
4. D. Mozaffarian, et al., “Plasma Phospholipid Long-Chain Omega-3 Fatty Acids and Total and Cause-Specific Mortality in Older Adults: A Cohort Study,” Ann. Inter. Med. 158 (7), 515–525 (2013).
5. J.E. Chavarro, “A Prospective Study of Polyunsaturated Fatty Acid Levels in Blood and Prostate Cancer Risk,” Cancer Epidemiol Biomarkers Prev. 16 (7), 1364–1370 (2007).
6. Centers for Disease Control and Prevention, www.cdc.gov/
nchs/data/dvs/deaths_2010_release.pdf.
7. K.M. Szymanski, D.C. Wheeler and L.A. Mucci, “Fish Consumption and Prostate Cancer Risk: A Review and Meta-Analysis,” Am. J. Clin. Nutr. 92, 1223–1233 (2010).
8. P. Terry, et al., “Fatty Fish Consumption and Risk of Prostate Cancer,” Lancet 357, 1764–1766 (2001).
9. M.F. Leitzmann, et al., “Dietary Intake of n-3 and n-6 Fatty Acids and the Risk of Prostate Cancer,” Am. J. Clin. Nutr. 80, 204–216 (2004).
10. K. Mina, L. Fritschi and K.C. Johnson, “An Inverse Association Between Preserved Fish and Prostate Cancer: Results From a Population-Based Case-Control Study in Canada,” Nutrition and Cancer 60, 222–226 (2008).
11. M.M. Epstein, et al., “Dietary Fatty Acid Intake and Prostate Cancer Survival in Orebro County, Sweden,” Amer. J. Epidemiol. 176, 240–252 (2012).
12. V. Fradet, et al., “Dietary Omega-3 Fatty Acids, Cyclooxygenase-2 Genetic Variation, and Aggressive Prostate Cancer Risk,” Clin. Cancer Res. 15, 2559–2566 (2009).
13. World Foundation of Urology NGO, “Prostate Cancer,” www.prostatecancerprevention.net/index.php?p=prostate-cancer, accessed Aug. 22, 2013.
Published in WholeFoods Magazine, October 2013










