For 90 years, vitamin E research has produced prolific and notable discoveries, including isolation from plants, chemical identifications and total syntheses. Until the last few decades, however, attention has been given mostly to the biological activities and underlying mechanisms of alpha-tocopherol, while more than one-third of all vitamin E tocotrienol research over the last 30 years was published in the last three years (2009–2011) (Figure 10). The thriving field of tocotrienol research gave ample ground for holding the 2nd International Tocotrienol Symposium (Long Beach, CA; April 29, 2012), where researchers presented their latest tocotrienol discoveries covering research areas that include cancer, skin health, radiation countermeasures, cognitive impairment, longevity and bone health, among others.
Barrie Tan, Ph.D., who has discussed with us the various benefits of vitamin  E tocotrienols over the last few months, was the event’s lead organizer and will give us a comprehensive review of the research presented at the symposium.
E tocotrienols over the last few months, was the event’s lead organizer and will give us a comprehensive review of the research presented at the symposium.
Dr. Tan earned his doctorate in chemistry with emphasis on biochemistry at the University of Otago, New Zealand. He later became a professor of chemistry and food science/nutrition at the University of Massachusetts, Amherst. His research expertise includes lipid-soluble materials such as carotenoids, tocotrienols/tocopherols, CoQ10, omega-3s and cholesterol. He was the first to introduce tocotrienol’s benefits to the nutrition industry. He founded American River Nutrition Inc. in 1998 and developed the first-ever tocopherol-free tocotrienol product derived from annatto beans. Today, the focus of his research is on lipid-soluble nutrients that have an impact on chronic and degenerative conditions. Aside from being the organizer of the 2nd International Tocotrienol Symposium in conjunction with the 103rd Annual Meeting of the American Oil Chemists’ Society, Dr. Tan was selected to be the senior editor of a compilation with broad focus on tocotrienol, Tocotrienols: Vitamin E Beyond Tocopherols (AOCS & CRC Press; October 2012). The publication of this book coincides well with our fourth and last interview with Dr. Tan, who will highlight the tocotrienol symposium where much of the detailed studies are documented.
Passwater: Dr. Tan, how did the planning of this second tocotrienol symposium come about?
Tan: As you probably gathered from the previous three parts of our interview, there has been a lot of new research on vitamin E tocotrienols. In fact, I like to refer to tocotrienol as the 21st Century vitamin E! In the four decades immediately following the discovery of vitamin E alpha-tocopherol by Drs. Herbert M. Evans and Katherine S. Bishop in 1922 (127), the main vitamin E function studied was its antioxidative properties (Figure 10). Antioxidation research continued throughout the 1970s and 1980s, and then diminished in the 1990s while tocotrienol studies picked up. Currently, the main areas of tocotrienol research are in cancer, cardiovascular disease and diabetes, while antioxidants are also making a comeback. Most recently, other research groups have sparked interest in tocotrienol’s ability to fight aging, counter radiation, decrease and reverse bone loss, and delay cognitive decline.
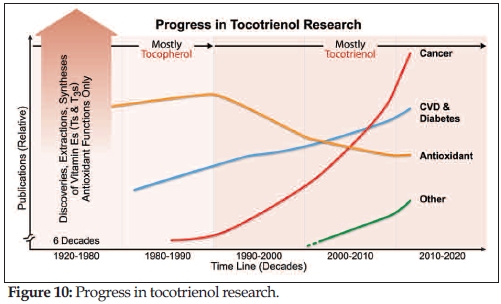
With all these studies happening independently across the globe, I knew that the time for a “meeting of the minds” had come. It was a long overdue symposium, as the first tocotrienol symposium was a half-day national meeting held six years ago at AOCS’s 97th annual meeting in 2006 (Saint Louis, MO). The 2012 talks and poster presentations served as symposium high points, but the goal was also to represent Tocotrienols: Vitamin E Beyond Tocopherols, Second Edition (October 2012) and to highlight the Emerging Science of tocotrienols. It was a distinct honor to see this 2nd International Tocotrienol Symposium coming to fruition, and I believe that scientists and attendees alike took away a rich exchange of ideas.
Passwater: In addition to delving into the latest research, was some background review on tocotrienols covered during the symposium?
Tan: Most speakers reviewed the basic differences between tocopherols and tocotrienols, including similarities in their configurations that make both molecules antioxidants, as well as structural differences that contribute to tocotrienol’s superior benefits in other areas such as cardiovascular health and cancer. While I began the symposium with a brief review of tocotrienol sources and the alpha-tocopherol interference issue, other speakers focused on tocotrienol’s mechanism and its recently confirmed safety for human usage.
Passwater: We talked about the alpha-tocopherol interference issue in Part 3 of the interview (September 2012), but could you briefly review this topic for readers who are just now joining us?
Tan: Certainly. Although alpha-tocopherol is  still the most supplemented form of vitamin E, an increasing mound of evidence shows that it not only interferes with tocotrienol benefits, but also has some potential side effects by itself. When taken at the same time as tocotrienol, only alpha-tocopherol has a right-of-passage through the gut due to the presence of a specific transport protein (alpha-tocopherol transport protein, ATTP) that facilitates its carriage through the body. Therefore, the absorption of tocotrienol becomes a challenge in the presence of alpha-tocopherol (128, 129).
still the most supplemented form of vitamin E, an increasing mound of evidence shows that it not only interferes with tocotrienol benefits, but also has some potential side effects by itself. When taken at the same time as tocotrienol, only alpha-tocopherol has a right-of-passage through the gut due to the presence of a specific transport protein (alpha-tocopherol transport protein, ATTP) that facilitates its carriage through the body. Therefore, the absorption of tocotrienol becomes a challenge in the presence of alpha-tocopherol (128, 129).
Moreover, alpha-tocopherol compromises tocotrienol’s ability to reduce cholesterol (130, 131) and fight cancer (132, 133), while increasing the breakdown of tocotrienols in the body (134). By itself, alpha-tocopherol may also lead to premature breakdown of prescription medications (135, 136), increased cholesterol (130, 131) and blood pressure (137), decreased bone mass (138) and increased risk of prostate cancer in healthy men (139). In addition, alpha-tocopherol decreases tocotrienol’s antioxidant potential (140). In previous clinical studies using different compositions of tocotrienol mixtures, those that contained about 25% or more alpha-tocopherol mainly did not work for cholesterol reduction (130, 131, 141–146), while those that contained about 10% or less alpha-tocopherol in tocopherol–tocotrienol mixtures generally worked (131, 147–156). This observation leads me to conclude that future clinical trials should have at least four study groups, including a control, tocotrienol, alpha-tocopherol, and tocotrienol plus alpha-tocopherol group.
Passwater: I look forward to seeing the results of this new generation of vitamin E clinical trials! For now, let’s explore how tocotrienols work. You mentioned that some speakers presented insights into tocotrienol’s mechanism.
Tan: Yes, two speakers apprised us on tocotrienol’s mechanism, particularly its effect on the body’s cholesterol production, also known as the mevalonic pathway.
Dr. Huanbiao Mo of Texas Woman’s University (Denton, TX), a co-organizer of the symposium, explained how the cholesterol biosynthesis pathway has a direct effect on carcinogenesis. He noted that tumor cells produce much more cholesterol than regular cells. The cholesterol-reducing drug statin has been shown to inhibit cancer growth, and in a similar fashion, tocotrienols suppress the tumor. Unfortunately, statins, although very effective in reducing cholesterol, cannot discriminate between cancer and normal cells, and hence toxicity is associated with their use. Tocotrienols, on the other hand, are selective when it comes to tumor cells. During cholesterol synthesis, an intermediate called farnesol is produced, from which cholesterol and many other important proteins are derived. When farnesol is too high in cells, it signals the cholesterol-producing enzyme, HMG-CoA reductase (or 3-hydroxy-3-methyl-glutaryl-CoA reductase) to dial down cholesterol production. Tocotrienols mimic farnesol and block cholesterol when too much is produced, as is the case in cancer cells. Dr. Mo also showed that statins and delta-tocotrienol work synergistically in melanoma (157), and that delta-tocotrienol efficiently suppresses pancreatic cancer cells (158).
Dr. Russell DeBose-Boyd of the University of Texas Southwestern Medical Center (Dallas, TX) presented more detail on the cholesterol biosynthesis pathway. Coincidentally, Dr. DeBose-Boyd is part of the lab group of Joseph Goldstein and Michael Brown, who were Nobel Prize recipients for their discovery of the LDL receptor in 1985. In his presentation, Dr. DeBose-Boyd described how the mevalonic pathway is subject to feedback regulation, and is controlled by products of the pathway that are important precursors of hormones and vitamins. These precursors, with the help of the membrane proteins Insig-1 and Insig-2, stimulate the degradation of HMG-CoA reductase. Tocotrienols, and especially delta- and gamma-tocotrienol, mimic the precursors, thereby downregulating HMG-CoA reductase. In addition, tocotrienol degrades the cholesterol-producing enzyme in the presence of Insig proteins (159). His explanation of how tocotrienol works to inhibit cholesterol is compelling and elegant, and it is as fundamental as it gets.
Passwater: This is quite novel. Interestingly, this mechanism was shown 20 years ago (160, 161), but with less precision. This really is unequivocal proof of delta- and gamma-tocotrienol effects in lowering cholesterol. You mentioned that tocotrienol safety was also addressed during the symposium?
Tan: Dr. Alexander Schauss, senior director of AIBMR Life Sciences, Inc. (Puyallup, WA), prepared a fantastic talk on tocotrienol safety. Dr. Schauss works closely with a U.S. Food and Drug Administration (FDA) expert panel to determine if a nutritional supplement or food ingredient should receive Generally Recognized as Safe (or GRAS) status. Criteria for GRAS affirmation include a clear picture of the ingredient’s chemistry and characteristics, satisfactory toxicological evaluations, an estimation of the current dietary intake, clarification on the intended conditions of use and an evaluation of the population that will be consuming the ingredient. In the past, GRAS affirmations were awarded by the FDA only, but it could often take years before an FDA response was received. Due to its work overload as a government agency, the FDA passed a rule that allows expert panels to perform GRAS evaluations (also known as self-affirmed GRAS).
During his own research on tocotrienols for a GRAS self-affirmation, Dr. Schauss found that Americans currently obtain about 4-8 mg of tocotrienols per day from the diet, while 25–205 mg daily dosages can be found in supplements available to the public. Despite the broad use of tocotrienol supplements, no adverse events have been reported to the FDA (via MedWatch) or the Centers for Disease Control and Prevention, which is truly remarkable and speaks volumes about the safety of this ingredient. A review of the scientific evidence proved uneventful for both in vitro and in vivo toxicology studies, and no genetic mutations or birth defects were found in reproductive and developmental toxicity studies. Authors of more than a dozen human clinical trials with tocotrienol reported no adverse events except transient effects in one study (162). Palm tocotrienols received FDA GRAS status in 2010, while annatto tocotrienols were self-affirmed GRAS in July 2010. “For annatto tocotrienols, the safe dose was determined to be 425 mg/day,” says Dr. Schauss, but believes that the safe dose of tocotrienols will likely increase in the near future due to a rise in consumption.
Passwater: With its track record of safety, tocotrienol will have a great future. Did any of the speakers update the audience on tocotrienol’s cardiovascular benefits?
Tan: There was one presentation that covered tocotrienol’s role in atherosclerosis. Dr. Hapizah Nawawi, a cardiologist from UTMara (Malaysia), reviewed in vitro and in vivo effects of tocotrienol on atherosclerosis and its risk factors. Most notably, tocotrienol suppressed the binding of monocytes to the endothelium, one of the primary events in atherosclerotic development. Delta-tocotrienol worked best to reduce monocyte-endothelial binding, while alpha-tocopherol enhanced the binding. In general, pure tocotrienols, and especially gamma- and delta-tocotrienol, were more potent than tocopherol-tocotrienol mixtures, particularly in reducing various pro-inflammatory signaling molecules. Tocopherol, on the other hand, inhibited the various benefits of tocotrienol. A tocopherol–tocotrienol mixture in atherosclerotic rabbits significantly reduced an adhesion molecule involved in monocyte-endothelial binding and reduced the atherosclerotic plaque. This mixture did not have an effect on the rabbits’ lipid levels, while tocotrienols alone reduced total and LDL cholesterol by approximately 50% at low doses. Dr. Nawawi’s study clearly underscores the negative impact of alpha-tocopherol on the positive anti-atherosclerotic effects of tocotrienols.
Passwater: That is a remarkable reduction in lipid levels. Did other studies corroborate these results?
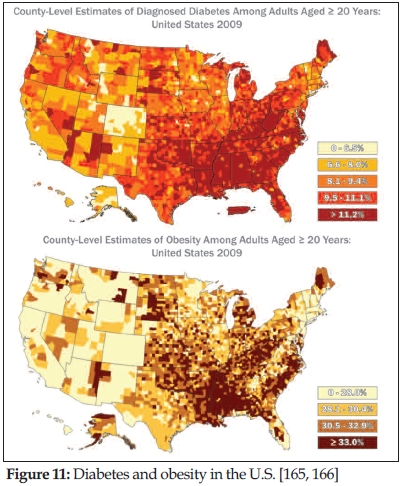
Tan: Dr. Lindsay Brown of the University of Queensland (Brisbane, Australia) presented his study results in a rat model, which also showed improved plasma lipid profiles. His animal model, however, was specifically designed for metabolic syndrome, which was induced in the rats using a high-fat, high-carb diet just like in human diets. It is of interest to note that Dr. Brown did not use genetically bred diabetic rats. In his study, animals received 120 mg/kg body weight of annatto tocotrienol without side effects. Following supplementation for 16 weeks, animals had improved cardiovascular function, normalized blood pressure, reduced heart stiffness and improved left ventricular structure (i.e., the part of the heart that pumps blood into the aorta). Glucose tolerance and handling also improved, as did insulin sensitivity. Remarkably, tocotrienol supplementation decreased fat deposition. Obesity itself is a hallmark of metabolic syndrome (Figure 11), and—as Dr. Brown mentioned—a disorder with low-grade chronic inflammation. Tocotrienols also appear to improve liver function and structure, with no inflammatory cells found in this organ as compared to the control. This may be of particular importance in non-alcoholic fatty liver disease, which is an increasing concern in diabetics (163) and an indication tocotrienol is beginning to have an impact on (164). Dr. Brown concludes that the inhibition of inflammation is a key target in tocotrienol’s effect on metabolic syndrome.
Passwater: Tocotrienol seems to play a prominent role in the fight against inflammation.
 Tan: Yes. Another speaker, Dr. Bharat Aggarwal of the University of Texas M.D. Anderson Cancer Center (Houston, TX) confirmed this in his review of tocotrienol’s anti-inflammatory properties. Inflammatory risk factors include smoking, sun exposure, obesity, stress, and infection. Inflammation is a chronic disease, and can lead to more serious conditions such as cancer. According to Dr. Aggarwal, cancer, for the most part, can be prevented by lifestyle changes, while only 5–10% of cancer occurrences can be attributed to genes. Unfortunately, cancer research funds are mismanaged, with 90–95% of funds being spent on the small portion of cancers attributed to genetics. Nuclear factor kB (NFkB) is a protein complex that controls the transcription of DNA. Dr. Aggarwal’s hypothesis for his cancer studies is that since NFkB is a major mediator of inflammation in most chronic diseases and cancer, an agent that could inhibit NFkB would delay or prevent the onset of such diseases. In order to suppress NFkB safely, chronic treatment would be required with a need to dial down multiple genes. Tocotrienols were found to target NFkB by multiple cell signaling pathways, and can be used safely for a prolonged period of time. While delta-tocotrienol is most active in suppressing NFkB, alpha-tocopherol opposes tocotrienol’s effect. Dr. Aggarwal also confirmed—along the lines of Dr. Mo and Dr. DeBose-Boyd—that HMG-CoA reductase is involved in the suppression of NFkB.
Tan: Yes. Another speaker, Dr. Bharat Aggarwal of the University of Texas M.D. Anderson Cancer Center (Houston, TX) confirmed this in his review of tocotrienol’s anti-inflammatory properties. Inflammatory risk factors include smoking, sun exposure, obesity, stress, and infection. Inflammation is a chronic disease, and can lead to more serious conditions such as cancer. According to Dr. Aggarwal, cancer, for the most part, can be prevented by lifestyle changes, while only 5–10% of cancer occurrences can be attributed to genes. Unfortunately, cancer research funds are mismanaged, with 90–95% of funds being spent on the small portion of cancers attributed to genetics. Nuclear factor kB (NFkB) is a protein complex that controls the transcription of DNA. Dr. Aggarwal’s hypothesis for his cancer studies is that since NFkB is a major mediator of inflammation in most chronic diseases and cancer, an agent that could inhibit NFkB would delay or prevent the onset of such diseases. In order to suppress NFkB safely, chronic treatment would be required with a need to dial down multiple genes. Tocotrienols were found to target NFkB by multiple cell signaling pathways, and can be used safely for a prolonged period of time. While delta-tocotrienol is most active in suppressing NFkB, alpha-tocopherol opposes tocotrienol’s effect. Dr. Aggarwal also confirmed—along the lines of Dr. Mo and Dr. DeBose-Boyd—that HMG-CoA reductase is involved in the suppression of NFkB.
Passwater: Were there any presentations of clinical studies validating the anti-inflammatory properties of tocotrienols?
Tan: I would be amiss if I did not mention Dr. Asaf Qureshi of the University of Missouri (Kansas City, MO) here, whom I consider to be the “father” of tocotrienol functions. Dr. Qureshi was the first to show that tocotrienol has differentiated properties to tocopherol, and that it is superior. Cholesterol reduction by tocotrienol was his first discovery. I had initially invited him to speak precisely on “Tocotrienol’s Anti-Inflammatory Properties in Humans,” but a schedule conflict prevented him from attending the symposium.
Dr. Qureshi has conducted systematic cell line and animal studies, as well as human trials to tease out the anti-inflammatory properties of tocotrienol (148, 153, 167–169). Dr. Qureshi demonstrated that alpha-, gamma- and delta-tocotrienols strongly inhibit the inflammatory response by looking at common factors associated with inflammation. Delta-tocotrienol was the most effective. Dr. Qureshi elegantly showed how tocotrienols increase the immune system’s ability to fight inflammation, and also enhance the body’s ability to discard unneeded or damaged proteins by modulating large protein complexes 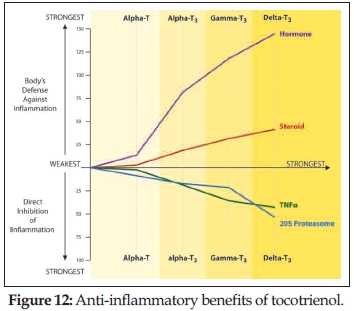 called proteasomes. At the same time, tocotrienols induce a hormone and, in turn, produce an anti-inflammatory steroid to block inflammation (Figure 12). In his most recent clinical trial, Dr. Qureshi gave hypercholesterolemic patients a supplement containing delta-tocotrienol along with other anti-inflammatory ingredients such as quercetin, resveratrol and riboflavin. He found that supplementation with these nutrients significantly reduced cardiovascular risk factors, including nitric oxide production—chronic expression of which is associated with several inflammatory conditions—and cholesterol (148). I would guess that Dr. Qureshi would have presented these studies at the symposium, all of which have been published recently.
called proteasomes. At the same time, tocotrienols induce a hormone and, in turn, produce an anti-inflammatory steroid to block inflammation (Figure 12). In his most recent clinical trial, Dr. Qureshi gave hypercholesterolemic patients a supplement containing delta-tocotrienol along with other anti-inflammatory ingredients such as quercetin, resveratrol and riboflavin. He found that supplementation with these nutrients significantly reduced cardiovascular risk factors, including nitric oxide production—chronic expression of which is associated with several inflammatory conditions—and cholesterol (148). I would guess that Dr. Qureshi would have presented these studies at the symposium, all of which have been published recently.
Passwater: Since tocotrienol is so effective on inflammatory conditions, I assume that there were several presentations on tocotrienol’s effect on cancer.
Tan: In fact, there were four presentations on tocotrienol’s anti-cancer effects. In current studies, tocotrienol appears to be effective against several deadly cancers (Figure 13). Dr. Francesco Galli of the University of Perugia (Italy) highlighted the superior function of delta- and gamma-tocotrienols for breast cancer in cell-line and animal studies (170). His studies involve the most severe prognosis of breast cancer, which is the overexpression of the HER-2 oncogene. Cells overexpressing this particular gene responded particularly well to delta-tocotrienol and annatto tocotrienol, resulting in cancer cell death. The mechanism appears to involve the production of reactive oxygen species or pro-oxidants in the cancer cells. There was also a dramatic uptake of delta-tocotrienol into cell membranes. In a mouse study, delta-tocotrienol and annatto tocotrienol reduced tumor size and metastases, while histology confirmed that the tocotrienol is effectively taken up into the tumor (171).
Apoptosis (or programmed cell death) is not the only mode of action tocotrienol has on cancer. Dr. Teruo Miyazawa of the Tohoku University (Sendai, Japan) spoke on the anti-angiogenic properties of tocotrienol. Dr. Miyazawa was the first to show that tocotrienol has anti-angiogenic properties. Angiogenesis is a principal factor in tumor growth and diabetic retinopathy. In mice, angiogenesis was prevented with low-dose oral tocotrienol supplementation (173). Tocotrienols employ three strategies to combat tumor angiogenesis. First, tocotrienols inhibit the angiogenic blood vessel formation directly by suppressing the activation of certain proteins that would normally encourage angiogenesis to occur. Second, tocotrienol inhibits growth factors that would otherwise lead to aberrant blood vessel growth. In this instance, tocotrienol is highly selective, and suppresses the secretion of growth factors under low oxygen conditions only, as commonly present in cancer cells. Delta-tocotrienol also suppressed NFkB expression in low-oxygen cancer cells, but not in normal healthy cells. Finally, tocotrienol has a direct effect on cancer by causing apoptosis, as Dr. Galli showed previously. Dr. Miyazawa went on to share with us some clinical trial results of tocotrienol’s effect on breast cancer. The study enrolled Japanese women with stage 2-4 breast cancer that were being treated with 25 days of radiotherapy along with hormone medicine and 500–1,000mg/day tocotrienols for five months. During the study, tumor size was reduced, and metastasis significantly prevented, while lymph node swelling was also normalized.
Passwater: It is great to hear of clinical cancer trials involving tocotrienols. Past clinical trials with vitamin E used mainly alpha-tocopherol, and unfortunately the results left much to be desired for.
Tan: One of our speakers, Dr. William Stone of the East Tennessee State University (Johnson City, TN) actually addressed one of these poorly publicized studies, and suggested a preferred model for testing tocotrienol’s effect in prostate cancer, the most common cancer in American men and the second leading cause of cancer deaths after lung cancer. Dr. Stone’s review of the Selenium and Vitamin E Cancer Prevention Trial (SELECT) shows that we have a long way to go in designing proper clinical trials for vitamin E. The SELECT study was a large-scale placebo-controlled clinical trial examining four patient groups: placebo, all-rac-alpha-tocopheryl acetate at 400 IU/day, selenium, and a selenium/alpha-tocopheryl acetate combo. The intervention trial ended in 2008 (174), but a 2011 follow-up survey of the patients showed a 17% increase in prostate cancer in the alpha-tocopheryl acetate group [139]. The conclusion statement of the study, “vitamin E significantly increased the risk of prostate cancer among healthy men,” was clearly misguided. Instead, the researchers and media coverage should have specified that the supplement given was not just a general “vitamin E,” but 400 IU/day of synthetic alpha-tocopheryl acetate.
Extrapolating clinical findings with one isoform of vitamin E casts unjustified suspicion on all of its other isoforms and their potential clinical uses. Future clinical trials must be well-designed and cost-effective, not like the SELECT that cost approximately $140 million. From a design perspective, the SELECT trial did not utilize the right vitamin E isomer, despite ample evidence supporting the anti-cancer effects of gamma-tocopherol and tocotrienols (175–182). In Dr. Stone’s own research, delta- and gamma-tocotrienol potently reduced prostate cancer cell line viability even at low doses, whereas alpha-tocopherol was least effective (175). Cellular uptake of delta- and gamma-tocotrienol into prostate cancer cells was also much greater, while alpha-tocotrienol was not absorbed. In colorectal cancer cells, on the other hand, alpha-tocopherol was observed to attenuate tocotrienol’s anti-cancer effect by decreasing the cellular uptake of delta-tocotrienol (133).
Dr. Stone’s targeted approach to a tocotrienol prostate chemoprevention trial would be to first identify a high-risk population, and then identify biomarkers that predict which individuals would be good responders. A high-risk population may be men with high cholesterol, since it is well documented that high cholesterol is associated with increased risk of prostate cancer (183–186), and prostate cancer cells accumulate cholesterol to spur their growth (187–189). The cholesterol-lowering drug statin has been shown to target cholesterol in prostate cancer in vitro and in vivo (190), and therefore men taking statins for high LDL cholesterol could be good responders in a future prostate cancer trial. Since tocotrienols and statins were shown to have synergistic anti-cancer benefits (191, 192), Dr. Stone suggests that the two be used in combination for prostate cancer. Moreover, to reduce study costs, he proposes to scan patients for a biomarker called PIN (prostatic intraepithelial neoplasia), which was shown to be reduced with dietary mixed tocotrienols (193).
I believe that tocopherol-free tocotrienol trials—using either gamma-tocotrienol and delta-tocotrienol mixtures or gamma-tocotrienol—are in the works for patients with breast or prostate cancer.
Passwater: If I recall correctly, there is currently a very compelling clinical trial on delta-tocotrienol’s effect on pancreatic cancer, and you alluded to it in our first interview four years ago.
Tan: Dr. Mokenge Malafa, a GI tract surgeon of the Moffitt Cancer Center and the University of Southern Florida (Tampa, FL), is the principal investigator of this clinical study with the goal to develop delta-tocotrienol for pancreatic cancer prevention. Pancreatic cancer is the most lethal of all cancers, with only a 5% survival rate. The problem with this type of cancer is that the pancreas is buried deep in the body, making it difficult to detect. When symptoms are noticed, such as dark urine, light stool, pancreatitis, abdominal and back pain, weight loss, loss of appetite, new onset diabetes, or painless jaundice, it is usually late stage.
Few people know that the pancreas consists of two different parts: one with vitamin A-containing cells (ductile) and the other with islet cells. The latter is responsible for insulin and hormone production, as well as nutrient metabolism. When defective, it can result in diabetes mellitus or metabolic syndrome. This is also the part of the pancreas where less severe tumors, or endocrine tumors, can develop, as was the case with Apple computer founder, Steve Jobs. The more serious type of pancreatic cancer occurs in the ductile cells, which is being studied by Dr. Malafa’s group. The medium survival rate for patients diagnosed with pancreatic cancer is disturbingly short: only two years, even for someone diagnosed with stage I. Hence, early diagnosis and prevention strategies are key. Of pancreatic cancer incidents, 85% are sporadic, 10% familial (such as was the case with former president Jimmy Carter’s family) and 5% are known as genetic syndromes. The greatest genetic risk for developing pancreatic cancer is for those with a condition called Peutz-Jeghers syndrome, in which a person develops intestinal polyps. Other genetic risk factors are hereditary pancreatitis and being a carrier of the oncogene BRCA2 present in some breast and ovarian cancers.
Some years ago, when I first met Dr. Malafa, he was looking to identify a food bioactive for application in pancreatic cancer prevention as part of an NIH grant. I suggested the use of delta-tocotrienol, but NIH insisted on a comparison of all vitamin E isomers. Lo and behold, delta-tocotrienol turned out to be the most effective of the E isomers against pancreatic cancer, closely followed in potency by gamma-tocotrienol. In Dr. Malafa’s preliminary studies, delta-tocotrienol inhibited the pancreatic tumor, and sufficient levels reached the pancreas and especially the tumor, while far less was detected in the blood (194). In his animal model, Dr. Malafa also showed that delta-tocotrienol increased survival and weight gain, while delaying the progression of pancreatic lesions. Tocotrienol’s mode of action in this case was programmed cell death. The preliminary findings led to a phase I clinical trial designed as a proof-of-concept (195). A total of 17 patients who were eligible for surgical pancreatic tumor removal participated, and received different doses of delta-tocotrienol with dosages ranging from 200 to 3,200mg/day. The primary endpoint of phase I was to find an effective dose for a phase II trial and to test the safety and tolerability of delta-tocotrienol. Even at the highest dose of 3,200mg, which according to Dr. Malafa was 5.6 times the predicted effective dose, no adverse effects were reported, and there was absolutely no toxicity from delta-tocotrienol supplementation. At 200–600mg doses, Dr. Malafa’s team already observed evidence of programmed cell death. I look forward to seeing this research move forward to phase II and III designs.
Passwater: Dr. Malafa has presented some encouraging data in the fight against this devastating disease. Aside from cancer, do tocotrienols have applications in other chronic or aging diseases?
Tan: Dr. Yoshikazu Nishikawa of Osaka City University (Japan) presented some novel findings on aging. He has been working with nematodes, and has shown that tocotrienols extend the life of these simple animals. Nematodes have a lifespan of only three weeks, and are therefore excellent subjects for studying biological aging, also known as senescence. Dr. Nishikawa’s first objective was to find out whether nutraceuticals can work in the worms. He noticed that nematodes’ host defense weakens over time, with old worms being sensitive to opportunistic infections whereas younger ones were not. Dr. Nishikawa treated worms with tocotrienols and found that delta-tocotrienol worked best in increasing the lifespan of nematodes, followed by gamma-tocotrienol and then alpha-tocotrienol. Tocotrienol’s effect on senescence was not due to caloric restriction, but rather the enhancement of the worms’ host defense, where tocotrienol-supplemented worms were resistant to opportunistic infections and showed evidence of reduced stress. Importantly, alpha-tocopherol reduced tocotrienol’s prolongevity effect. In Dr. Nishikawa’s anti-aging studies, we find yet another instance of alpha-tocopherol interference.
Passwater: It should be interesting to see how this anti-aging research can be translated from worm to human. Longer life is associated with other age-related diseases as well. Were any of these presented during the symposium?
Tan: We had two very interesting presentations related to this subject, one on cognitive impairment and Alzheimer’s disease, the other on bone health and anti-osteoporosis. Dr. Francesca Mangialasche, researcher at the Karolinska Institute (Stockholm, Sweden) and geriatrician, gave us some new insights on vitamin E’s role in cognition and related diseases (196). Current evidence suggests that alpha-tocopherol was inconsistent at best in protecting from mild cognitive impairment or Alzheimer’s disease, whereas vitamin E from the diet has shown some protective effects with increased intake. In a clinical study testing the tocopherol and tocotrienol levels in plasma of patients with Alzheimer’s disease or mild cognitive impairment, researchers found that vitamin E levels were reduced in subjects with the disease. Dr. Mangialasche reviewed both a Swedish and a Finnish population-based longitudinal study, where the Swedish study showed higher levels of tocopherols and tocotrienols to be associated with a reduced risk by as much as 50% of Alzheimer’s disease. Alpha- and beta- tocopherols and tocotrienols were the most protective in this study. In the Finnish study, gamma-tocopherol was the most protective. In a test that explores global cognition, a greater amount of total tocotrienols was associated with better cognition scores. Dr. Mangialasche concludes both tocopherols and tocotrienols are important for Alzheimer’s disease protection, but prevention and treatment strategies need to be refined in future clinical trials by testing all vitamin E isomers.
Until now, tocopherols (besides alpha-tocopherol) in general, and tocotrienols in particular, have never been clearly associated with cognition in a population study. It is not proof, but it is strongly suggestive. With further refinements, as Dr. Mangialasche indicated, we will be able to tease out the effectiveness of vitamin E molecules. Until then, they are a reasonable insurance policy.
 Passwater: Dr. Mangialasche presented some long-needed updates on vitamin E’s neuroprotective roles. I have not yet heard about the bone health research. How could tocotrienol work in osteoporosis?
Passwater: Dr. Mangialasche presented some long-needed updates on vitamin E’s neuroprotective roles. I have not yet heard about the bone health research. How could tocotrienol work in osteoporosis?
Tan: The bone health research was presented by Dr. Ima Soelaiman, an endocrinologist and bone specialist at the Universiti Kebangsaan Malaysia (Kuala Lumpur, Malaysia), and is based on tocotrienol’s effect as an antioxidant. It is known that free radicals lead to increased bone loss and bone resorption, with an imbalance between oxidant and antioxidant in postmenopausal osteoporosis. Antioxidants, in turn, may prevent osteoporosis. Dr. Soelaiman utilized a rat model, which is similar in bone anatomy to humans. Tocotrienol prevented changes in nicotine-induced osteoporosis and increased bone calcium content. The tocopherol–tocotrienol mixture used in this study contained 88% tocotrienols and only 12% tocopherols. Tocotrienol treatment for two months reversed the adverse effects of nicotine, and the bone structure of the treated group was even better than that of the non-osteoporosis control group! Gamma-tocotrienol by itself worked even better than the mixture or alpha-tocopherol. Tocotrienol also improves resistance to fracture and strength of the fractured osteoporotic bone after healing has occurred. In rats, tocotrienol seems to work better than estrogen replacement therapy for osteoporosis (197, 198). Dr. Soelaiman also designed animal studies for estrogen-deficient osteoporosis using annatto tocotrienol alone and in combo with lovastatin with very encouraging results, and these data will be published in the near future.
Passwater: The 2nd International Tocotrienol Symposium has brought forth some fascinating research! Are there other studies we have not yet mentioned?
Tan: There is one other speaker whose presentation I would like to review, and he covers a completely different subject. Dr. Venkataraman Srinivasan of the Armed Forces Radiobiology Research Institute (AFRRI; Bethesda, MD) spoke on tocotrienol’s potential as a radiation countermeasure agent. This research had been classified for some years, and has only recently been published. AFRRI is concerned with the biological effects of radiation on both military personnel (such as first responders) as well as civilian scientists. They also represent a medical emergency response team and hence both prophylaxis and therapy are important treatment strategies they target to increase survival after radiation exposure. Since radiation research cannot be carried out in humans, the FDA passed what is known as the “animal rule,” where animals are sufficient as a model to confirm the suitability of a potential anti-radiation drug.
AFRRI focuses on subclinical effects of radiation exposure that can be controlled by drugs or preventive measures, which is the stem cell system in the case of acute radiation syndrome. Side effects of increased radiation that respond only poorly to intervention include gastrointestinal and cardiovascular symptoms. A promising drug must pass simple toxicity and basic radiation survival studies, and then enters optimization studies to determine the time and route of administration, as well as the dosage. The rationale for using tocotrienol in these studies was its high antioxidant potential. Both delta- and gamma-tocotrienol passed basic survival studies in a mouse model, and worked even at low dosages (199, 200). Delta-tocotrienol also restored white blood cells, neutrophils, lymphocytes and platelets more quickly. Route of administration was subcutaneous, since military personnel cannot take orals at the time of exposure because they have to wear masks. The optimum dose for delta- and gamma-tocotrienol appears to be 100–200 mg/kg in mice, and non-human primate studies will soon be started.
Passwater: Thank you, Dr. Tan. This was a great summary of  the meeting that brought together top physicians and scientists in tocotrienol research (Figure 14). Other presenters at the symposium were not reviewed here because of the need for brevity. I would like to let our readers know that most of the studies presented at the symposium will be reviewed in the soon-to-be published second edition of Tocotrienols: Vitamin E Beyond Tocopherols (Figure 15), while this book also covers additional topics by authors that did not attend the symposium.
the meeting that brought together top physicians and scientists in tocotrienol research (Figure 14). Other presenters at the symposium were not reviewed here because of the need for brevity. I would like to let our readers know that most of the studies presented at the symposium will be reviewed in the soon-to-be published second edition of Tocotrienols: Vitamin E Beyond Tocopherols (Figure 15), while this book also covers additional topics by authors that did not attend the symposium.
I expect that future research will continue to point to tocotrienol as the 21st Century Vitamin E (201, 202). WF
Dr. Richard Passwater is the author of more than 45 books and 500 articles on nutrition. Dr. Passwater has been WholeFoods Magazine’s science editor and author of this column since 1984. More information is available on his Web site, www.drpasswater.com.
References
1. Tan, B., Appropriate spectrum vitamin E and new perspectives on desmethyl tocopherols and tocotrienols. JANA, 2005. 8(1): p. 35-42.
2. Lee, R., Gamma-tocopherol metabolism and its relationship with alpha-tocopherol in humans., in The Antioxidant Vitamins C and E, L. Packer, et al., Editors. 2002, AOCS Press: Champaign, IL. p. 180-194.
3. Eitenmiller, R. and L. J., Analysis of tocopherols and tocotrienols in food., in Vitamin E: Food Chemistry, Composition, and Analysis. 2004, Marcel Dekker, Inc.: New York. p. 364-366.
4. Swanson, J.E., et al., Urinary excretion of 2,7, 8-trimethyl-2-(beta-carboxyethyl)-6-hydroxychroman is a major route of elimination of gamma-tocopherol in humans. J Lipid Res, 1999. 40(4): p. 665-71.
5. Sylvester, P. and A. Theriault, Role of tocotrienols in the prevention of cardiovascular disease and breast cancer. Curr Top in Nutra Res, 2003. 1(2): p. 121-136.
6. Wada, S., et al., Tumor suppressive effects of tocotrienol in vivo and in vitro. Cancer Lett, 2005. 229(2): p. 181-91.
7. Yano, Y., et al., Induction of cytotoxicity in human lung adenocarcinoma cells by 6-O-carboxypropyl-alpha-tocotrienol, a redox-silent derivative of alpha-tocotrienol. Int J Cancer, 2005. 115(5): p. 839-46.
8. Yang, C.S., N. Suh, and A.N. Kong, Does vitamin e prevent or promote cancer? Cancer Prev Res (Phila), 2012. 5(5): p. 701-5.
9. Klein, E.A., et al., Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA, 2011. 306(14): p. 1549-56.
10. Galli, F., et al., The effect of alpha- and gamma-tocopherol and their carboxyethyl hydroxychroman metabolites on prostate cancer cell proliferation. Arch Biochem Biophys, 2004. 423(1): p. 97-102.
11. Jiang, Q., et al., gamma-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc Natl Acad Sci U S A, 2004. 101(51): p. 17825-30.
12. McIntyre, B.S., et al., Antiproliferative and apoptotic effects of tocopherols and tocotrienols on preneoplastic and neoplastic mouse mammary epithelial cells. Proc Soc Exp Biol Med, 2000. 224(4): p. 292-301.
13. Wang, Y., et al., Vitamin E forms inhibit IL-13/STAT6-induced eotaxin-3 secretion by up-regulation of PAR4, an endogenous inhibitor of atypical PKC in human lung epithelial cells. J Nutr Biochem, 2011.
14. Li, G.X., et al., delta-tocopherol is more active than alpha - or gamma -tocopherol in inhibiting lung tumorigenesis in vivo. Cancer Prev Res (Phila), 2011. 4(3): p. 404-13.
15. Theriault, A., et al., Tocotrienol: a review of its therapeutic potential. Clin Biochem, 1999. 32(5): p. 309-19.
16. Elson, C.E., Suppression of mevalonate pathway activities by dietary isoprenoids: protective roles in cancer and cardiovascular disease. J Nutr, 1995. 125(6 Suppl): p. 1666S-1672S.
17. Song, B.L. and R.A. DeBose-Boyd, Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme a reductase stimulated by delta- and gamma-tocotrienols. J Biol Chem, 2006. 281(35): p. 25054-61.
18. Miyazawa, T., et al., Anti-angiogenic function of tocotrienol. Asia Pac J Clin Nutr, 2008. 17 Suppl 1: p. 253-6.
19. Nakagawa, K., et al., DNA chip analysis of comprehensive food function: inhibition of angiogenesis and telomerase activity with unsaturated vitamin E, tocotrienol. Biofactors, 2004. 21(1-4): p. 5-10.
20. Nesaretnam, K. and P. Meganathan, Tocotrienols: inflammation and cancer. Ann N Y Acad Sci, 2011. 1229(1): p. 18-22.
21. Mizushina, Y., et al., Inhibitory effect of tocotrienol on eukaryotic DNA polymerase lambda and angiogenesis. Biochem Biophys Res Commun, 2006. 339(3): p. 949-55.
22. Li, Y., et al., gamma-Tocotrienol inhibits angiogenesis of human umbilical vein endothelial cell induced by cancer cell. J Nutr Biochem, 2011. 22(12): p. 1127-36.
23. Sakai, M., et al., Apoptosis induction by gamma-tocotrienol in human hepatoma Hep3B cells. J Nutr Biochem, 2006. 17(10): p. 672-6.
24. Springett, G., et al., A phase I dose-escalation study of the safety, PK, and PD of vitamin E delta-tocotrienol administered to subjects with resectable exocrine neoplasia., in 102nd Annual Meeting of the American Association for Cancer Research. 2011: Orlando, FL.
25. Husain, K., et al., Vitamin E delta-tocotrienol levels in tumor and pancreatic tissue of mice after oral administration. Pharmacology, 2009. 83(3): p. 157-63.
26. Husain, K., et al., Vitamin E delta-tocotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF-kappaB activation in pancreatic cancer. Mol Cancer Ther, 2011. 10(12): p. 2363-72.
27. Hussein, D. and H. Mo, d-Delta-tocotrienol-mediated suppression of the proliferation of human PANC-1, MIA PaCa-2, and BxPC-3 pancreatic carcinoma cells. Pancreas, 2009. 38(4): p. e124-36.
28. Shin-Kang, S., et al., Tocotrienols inhibit AKT and ERK activation and suppress pancreatic cancer cell proliferation by suppressing the ErbB2 pathway. Free Radic Biol Med, 2011. 51(6): p. 1164-74.
29. American Cancer Society. Cancer Facts & Figures. 2011 [cited; Available from: www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-029771.pdf.
30. Zaiden, N., et al., Gamma delta tocotrienols reduce hepatic triglyceride synthesis and VLDL secretion. J Atheroscler Thromb, 2010. 17(10): p. 1019-32.
31. Heber, D., Tocotrienols and cholesterol homeostasis: Basic and clinical research perspectives, in 2nd International Conference on Tocotrienols & Chronic Diseases. 2011: Las Vegas, NV.
32. Serbinova, E.A. and L. Packer, Antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Methods Enzymol, 1994. 234: p. 354-66.
33. Packer, L., S.U. Weber, and G. Rimbach, Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling. J Nutr, 2001. 131(2): p. 369S-73S.
34. Muller, L., K. Theile, and V. Bohm, In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma. Mol Nutr Food Res, 2010. 54(5): p. 731-42.
35. Qureshi, A.A., et al., Isolation and identification of novel tocotrienols from rice bran with hypocholesterolemic, antioxidant, and antitumor properties. J Agric Food Chem, 2000. 48(8): p. 3130-40.
36. Goodell, J., The Fire Next Time, in Rolling Stone. 2011. p. 35-38.
37. Armed Forces Radiobiology Research Institute. Radiation Countermeasures. 2011 [cited 6/14/2011]; Available from: http://www.usuhs.mil/afrri/research/rcp.htm.
38. Kulkarni, S., et al., Gamma-tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation. Radiat Res, 2010. 173(6): p. 738-47.
39. Li, X.H., et al., Delta-tocotrienol protects mouse and human hematopoietic progenitors from gamma-irradiation through extracellular signal-regulated kinase/mammalian target of rapamycin signaling. Haematologica, 2010. 95(12): p. 1996-2004.
40. Ghosh, S.P., et al., Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int J Radiat Biol, 2009. 85(7): p. 598-606.
41. Satyamitra, M.M., et al., Hematopoietic Recovery and Amelioration of Radiation-Induced Lethality by the Vitamin E Isoform delta-Tocotrienol. Radiat Res, 2011. 175(6): p. 736-45.
42. Kaileh, M. and R. Sen, Role of NF-kappaB in the anti-inflammatory effects of tocotrienols. J Am Coll Nutr, 2010. 29(3 Suppl): p. 334S-339S.
43. Kannappan, R., et al., Tocotrienols fight cancer by targeting multiple cell signaling pathways. Genes Nutr, 2011. 7(1): p. 43-52.
44. Qureshi, A.A., et al., Tocotrienols inhibit lipopolysaccharide-induced pro-inflammatory cytokines in macrophages of female mice. Lipids Health Dis, 2011. 9(1): p. 143.
45. Mehat, M.Z., et al., Beneficial effects of vitamin E isomer supplementation on static and dynamic bone histomorphometry parameters in normal male rats. J Bone Miner Metab, 2010. 28(5): p. 503-9.
46. Michihara, A., et al., Delta-tocotrienol causes decrease of melanin content in mouse melanoma cells. J Health Sci, 2009. 55(2): p. 314-318.
47. Yap, W.N., et al., Gamma- and delta-tocotrienols inhibit skin melanin synthesis by suppressing constitutive and UV-induced tyrosinase activation. Pigment Cell Melanoma Res, 2010. 23(5): p. 688-92.
48. Nishikawa, Y., Prolongevity effects of tocotrienols in Caenorhabditis elegans, in 2nd International Tocotrienol Symposium. 2012: Long Beach, CA.
49. Azlina, M.F., M.I. Nafeeza, and B.A. Khalid, A comparison between tocopherol and tocotrienol effects on gastric parameters in rats exposed to stress. Asia Pac J Clin Nutr, 2005. 14(4): p. 358-65.
50. Brown, L., δ-Tocotrienol from annatto oil ameliorates metabolic syndrome developed in high carbohydrate, high fat-diet fed rats, in 2nd International Tocotrienol Symposium. 2012: Long Beach, CA.
51. Rink, C., et al., Tocotrienol vitamin E protects against preclinical canine ischemic stroke by inducing arteriogenesis. J Cereb Blood Flow Metab, 2011. 31(11): p. 2218-30.
52. Kuhad, A. and K. Chopra, Attenuation of diabetic nephropathy by tocotrienol: involvement of NFkB signaling pathway. Life Sci, 2009. 84(9-10): p. 296-301.
53. Anderson, S.L., J. Qiu, and B.Y. Rubin, Tocotrienols induce IKBKAP expression: a possible therapy for familial dysautonomia. Biochem Biophys Res Commun, 2003. 306(1): p. 303-9.
54. Anderson, S.L. and B.Y. Rubin, Tocotrienols reverse IKAP and monoamine oxidase deficiencies in familial dysautonomia. Biochem Biophys Res Commun, 2005. 336(1): p. 150-6.
55. Mangialasche, F., et al., Tocopherols and tocotrienols plasma levels are associated with cognitive impairment. Neurobiol Aging, 2011.
56. Mueller, A.M., B. Tan, and E.S. Stuart, Tocotrienol in the potential treatment of infectious disease., in Tocotrienol: Vitamin E beyond Tocopherol., R. Watson and V. Preedy, Editors. 2008, CRC Press. p. 343-359.
57. Mahalingam, D., et al., Effects of supplementation with tocotrienol-rich fraction on immune response to tetanus toxoid immunization in normal healthy volunteers. Eur J Clin Nutr, 2011. 65(1): p. 63-9.
58. Hafid, S.R., A.K. Radhakrishnan, and K. Nesaretnam, Tocotrienols are good adjuvants for developing cancer vaccines. BMC Cancer, 2010. 10: p. 5.
59. Wilankar, C., et al., Role of immunoregulatory transcription factors in differential immunomodulatory effects of tocotrienols. Free Radic Biol Med, 2011. 51(1): p. 129-43.
60. Ren, Z., et al., Dietary supplementation with tocotrienols enhances immune function in C57BL/6 mice. J Nutr, 2010. 140(7): p. 1335-41.
61. Chao, J.T., A. Gapor, and A. Theriault, Inhibitory effect of delta-tocotrienol, a HMG CoA reductase inhibitor, on monocyte-endothelial cell adhesion. J Nutr Sci Vitaminol (Tokyo), 2002. 48(5): p. 332-7.
62. Theriault, A., J.T. Chao, and A. Gapor, Tocotrienol is the most effective vitamin E for reducing endothelial expression of adhesion molecules and adhesion to monocytes. Atherosclerosis, 2002. 160(1): p. 21-30.
63. Naito, Y., et al., Tocotrienols reduce 25-hydroxycholesterol-induced monocyte-endothelial cell interaction by inhibiting the surface expression of adhesion molecules. Atherosclerosis, 2005. 180(1): p. 19-25.
64. Passwater, R.A., Health Benefits Beyond Vitamin E Activity:Solving the Tocotrienol Riddle
An Interview with Dr. Barrie Tan. Whole Foods Magazine, 2008(June/July 2008).
65. Black, T.M., et al., Palm tocotrienols protect ApoE +/- mice from diet-induced atheroma formation. J Nutr, 2000. 130: p. 2420-2426.
66. Qureshi, A.A., et al., Novel tocotrienols of rice bran inhibit atherosclerotic lesions in C57BL/6 ApoE-deficient mice. J Nutr, 2001. 131(10): p. 2606-18.
67. Patel, V., et al., Oral tocotrienols are transported to human tissues and delay the progression of the model for end-stage liver disease score in patients. J Nutr, 2012. 142(3): p. 513-9.
68. Levin, N., The trends, in Nutrition Business Journal. 2012. p. 50.
69. Associated Press. FDA outlines rules for nanotechnology in foods. 2012 [cited 4/20/12].
70. Schwellenbach, L.J., et al., The triglyceride-lowering effects of a modest dose of docosahexaenoic acid alone versus in combination with low dose eicosapentaenoic acid in patients with coronary artery disease and elevated triglycerides. J Am Coll Nutr, 2006. 25(6): p. 480-5.
71. Kooyenga, D.K., et al., Antioxidants modulate the course of carotid atherosclerosis: A four-year report., in Micronutrients and Health, K. Nesaretnam and L. Packer, Editors. 2001, AOCS Press: Illinois. p. 366-375.
72. Black, T.M., et al., Palm tocotrienols protect ApoE +/- mice from diet-induced atheroma formation. J Nutr, 2000. 130: p. 2420-2426.
73. Qureshi, A.A., et al., Novel tocotrienols of rice bran inhibit atherosclerotic lesions in C57BL/6 ApoE-deficient mice. J Nutr, 2001. 131(10): p. 2606-18.
74. Nawawi, H. Tocotrienols: The Underlying Anti-Atherosclerotic Mechanisms. in 2nd International Tocotrienol Symposium. April 29, 2012. Long Beach, CA.
75. Ikeda, S., et al., Dietary alpha-tocopherol decreases alpha-tocotrienol but not gamma-tocotrienol concentration in rats. J Nutr, 2003. 133(2): p. 428-34.
76. Khanna, S., et al., Delivery of orally supplemented alpha-tocotrienol to vital organs of rats and tocopherol-transport protein deficient mice. Free Radic Biol Med, 2005. 39(10): p. 1310-9.
77. Uchida, T., et al., Tissue distribution of alpha- and gamma-tocotrienol and gamma-tocopherol in rats and interference with their accumulation by alpha-tocopherol. Lipids, 2012. 47(2): p. 129-39.
78. Qureshi, A.A., et al., Dietary alpha-tocopherol attenuates the impact of gamma-tocotrienol on hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in chickens. J Nutr, 1996. 126(2): p. 389-94.
79. Khor, H.T. and T.T. Ng, Effects of administration of alpha-tocopherol and tocotrienols on serum lipids and liver HMG CoA reductase activity. Int J Food Sci Nutr, 2000. 51 Suppl: p. S3-11.
80. Shibata, A., et al., alpha-Tocopherol attenuates the cytotoxic effect of delta-tocotrienol in human colorectal adenocarcinoma cells. Biochem Biophys Res Commun, 2010. 397(2): p. 214-9.
81. Guthrie, N., et al., Inhibition of proliferation of estrogen receptor-negative MDA-MB-435 and -positive MCF-7 human breast cancer cells by palm oil tocotrienols and tamoxifen, alone and in combination. J Nutr, 1997. 127: p. 544S-548S.
82. Sontag, T.J. and R.S. Parker, Influence of major structural features of tocopherols and tocotrienols on their omega-oxidation by tocopherol-omega-hydroxylase. J Lipid Res, 2007. 48(5): p. 1090-8.
83. Brigelius-Flohe, R., Adverse effects of vitamin E by induction of drug metabolism. Genes Nutr, 2007. 2(3): p. 249-56.
84. Brigelius-Flohe, R., Induction of drug metabolizing enzymes by vitamin E. J Plant Physiol, 2005. 162(7): p. 797-802.
85. Khor, H.T., D.Y. Chieng, and K.K. Ong, Tocotrienols: A Dose-Dependent Inhibitor for HMGCoA Reductase, in Nutrition, Lipids, Health, and Disease, A.S.H. Ong, E. Niki, and L. Packer, Editors. 1995, AOCS Press: Champaign, Illinois. p. 104-108.
86. Khor, H.T., D.Y. Chirng, and K.K. Ong, Tocotrienols inhibit HMG-CoA reductase activity in the guinea pig. Nutr. Res., 1995(15): p. 537-544.
87. Miyamoto, K., et al., Very-high-dose alpha-tocopherol supplementation increases blood pressure and causes possible adverse central nervous system effects in stroke-prone spontaneously hypertensive rats. J Neurosci Res, 2009. 87(2): p. 556-66.
88. Klein, E.A., et al., Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA, 2011. 306(14): p. 1549-56.
89. Campbell, S.E., et al., gamma-Tocotrienol induces growth arrest through a novel pathway with TGFbeta2 in prostate cancer. Free Radic Biol Med, 2011. 50(10): p. 1344-54.
90. Fujita, K., et al., Vitamin E decreases bone mass by stimulating osteoclast fusion. Nat Med, 2012.
91. Trias, A.M. and B. Tan, eds. Alpha-Tocopherol: A Detriment to Tocotrienol Benefits. 2 ed. Tocotrienols: Vitamin E Beyond Tocopherols, ed. B. Tan, R. Watson, and V. Preedy. 2012, AOCS/CRC Press.
92. Ju, J., et al., Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis, 2010. 31(4): p. 533-42.
93. Yu, W., et al., Anticancer actions of natural and synthetic vitamin E forms: RRR-alpha-tocopherol blocks the anticancer actions of gamma-tocopherol. Mol Nutr Food Res, 2009. 53(12): p. 1573-81.
94. Li, G.X., et al., delta-tocopherol is more active than alpha - or gamma -tocopherol in inhibiting lung tumorigenesis in vivo. Cancer Prev Res (Phila), 2011. 4(3): p. 404-13.
95. Tan, B., Appropriate spectrum vitamin E and new perspectives on desmethyl tocopherols and tocotrienols. JANA, 2005. 8(1): p. 35-42.
96. Handelman, G.J., et al., Oral alpha-tocopherol supplements decrease plasma gamma-tocopherol levels in humans. J Nutr, 1985. 115(6): p. 807-13.
97. Huang, H.Y. and L.J. Appel, Supplementation of diets with alpha-tocopherol reduces serum concentrations of gamma- and delta-tocopherol in humans. J Nutr, 2003. 133(10): p. 3137-40.
98. Papas, A., The Vitamin E Factor. First ed. 1999, New York: Harper Perennial. 395.
99. Traber, M.G., Vitamin E regulatory mechanisms. Annu Rev Nutr, 2007. 27: p. 347-62.
100. American Heart Association, 1998.
101. Graveline, D., Tocotrienols in combat: fighting the statin damage crisis, in Whole Foods Magazine. October 2011. p. 44-46.
102. Atkinson, J., R.F. Epand, and R.M. Epand, Tocopherols and tocotrienols in membranes: a critical review. Free Radic Biol Med, 2008. 44(5): p. 739-64.
103. O'Byrne, D., et al., Studies of LDL oxidation following alpha-, gamma-, or delta-tocotrienyl acetate supplementation of hypercholesterolemic humans. Free Radic Biol Med, 2000. 29(9): p. 834-45.
104. Neuzil, J., et al., Vitamin E analogues as inducers of apoptosis: implications for their potential antineoplastic role. Redox Rep, 2001. 6(3): p. 143-51.
105. Prasad, K.N. and J. Edwards-Prasad, Effects of tocopherol (vitamin E) acid succinate on morphological alterations and growth inhibition in melanoma cells in culture. Cancer Res, 1982. 42(2): p. 550-5.
106. Rama, B.N. and K.N. Prasad, Study on the specificity of alpha-tocopheryl (vitamin E) acid succinate effects on melanoma, glioma and neuroblastoma cells in culture. Proc Soc Exp Biol Med, 1983. 174(2): p. 302-7.
107. Swettenham, E., et al., Alpha-tocopheryl succinate selectively induces apoptosis in neuroblastoma cells: potential therapy of malignancies of the nervous system? J Neurochem, 2005. 94(5): p. 1448-56.
108. Turley, J.M., et al., Vitamin E succinate induces Fas-mediated apoptosis in estrogen receptor-negative human breast cancer cells. Cancer Res, 1997. 57(5): p. 881-90.
109. Turley, J.M., et al., Growth inhibition and apoptosis of RL human B lymphoma cells by vitamin E succinate and retinoic acid: role for transforming growth factor beta. Cell Growth Differ, 1995. 6(6): p. 655-63.
110. Wu, K., et al., Effect of vitamin E succinate on expression of TGF-beta1, c-Jun and JNK1 in human gastric cancer SGC-7901 cells. World J Gastroenterol, 2001. 7(1): p. 83-7.
111. Johnson, T.E., et al., Statins induce apoptosis in rat and human myotube cultures by inhibiting protein geranylgeranylation but not ubiquinone. Toxicol Appl Pharmacol, 2004. 200(3): p. 237-50.
112. Dudka, J., et al., Activity of NADPH-cytochrome P-450 reductase of the human heart, liver and lungs in the presence of (-)-epigallocatechin gallate, quercetin and resveratrol: an in vitro study. Basic Clin Pharmacol Toxicol, 2005. 97(2): p. 74-9.
113. Malafa, M.P., et al., Vitamin E inhibits melanoma growth in mice. Surgery, 2002. 131(1): p. 85-91.
114. Malafa, M.P. and L.T. Neitzel, Vitamin E succinate promotes breast cancer tumor dormancy. J Surg Res, 2000. 93(1): p. 163-70.
115. Quin, J., et al., Vitamin E succinate decreases lung cancer tumor growth in mice. J Surg Res, 2005. 127(2): p. 139-43.
116. Springett, G., et al. Delta-Tocotrienol in Subjects With Resectable Pancreatic Exocrine Neoplasia. in 2nd International Tocotrienol Symposium. April 29, 2012. Long Beach, CA.
117. Pimiento, J.M., et al. Vitamin E Delta-Tocotrienol Prevents Azoxymethane-Induced Colon Carcinogenesis Progression in Fisher-344 Rats. in 2nd International Tocotrienol Symposium. 2012. Long Beach, CA.
118. Husain, K., et al., Vitamin E delta-tocotrienol levels in tumor and pancreatic tissue of mice after oral administration. Pharmacology, 2009. 83(3): p. 157-63.
119. Husain, K., et al., Vitamin E delta-tocotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF-kappaB activation in pancreatic cancer. Mol Cancer Ther, 2011. 10(12): p. 2363-72.
120. Zu, K., L. Hawthorn, and C. Ip, Up-regulation of c-Jun-NH2-kinase pathway contributes to the induction of mitochondria-mediated apoptosis by alpha-tocopheryl succinate in human prostate cancer cells. Mol Cancer Ther, 2005. 4(1): p. 43-50.
121. Shklar, G., et al., Regression by vitamin E of experimental oral cancer. J Natl Cancer Inst, 1987. 78(5): p. 987-92.
122. Zhang, M., S. Altuwaijri, and S. Yeh, RRR-alpha-tocopheryl succinate inhibits human prostate cancer cell invasiveness. Oncogene, 2004. 23(17): p. 3080-8.
123. Yap, S.P., K.H. Yuen, and J.W. Wong, Pharmacokinetics and bioavailability of alpha-, gamma- and delta-tocotrienols under different food status. J Pharm Pharmacol, 2001. 53(1): p. 67-71.
124. Evans, H.M. and K.S. Bishop, On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science, 1922. 56: p. 650-651.
125. Qureshi, A.A., et al., The structure of an inhibitor of cholesterol biosynthesis isolated from barley. J Biol Chem, 1986. 261(23): p. 10544-50.
126. Eitenmiller, R. and L. J., Analysis of tocopherols and tocotrienols in food., in Vitamin E: Food Chemistry, Composition, and Analysis. 2004, Marcel Dekker, Inc.: New York. p. 364-366.
127(part 3).Qureshi, A.A., et al., Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF25) of rice bran in hypercholesterolemic humans. Atherosclerosis, 2002. 161(1): p. 199-207.
127(part 4). Evans, H.M. and K.S. Bishop, On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science, 1922. 56: p. 650-651.
128. Ikeda, S., et al., Dietary alpha-tocopherol decreases alpha-tocotrienol but not gamma-tocotrienol concentration in rats. J Nutr, 2003. 133(2): p. 428-34.
129. Uchida, T., et al., Tissue distribution of alpha- and gamma-tocotrienol and gamma-tocopherol in rats and interference with their accumulation by alpha-tocopherol. Lipids, 2012. 47(2): p. 129-39.
130. Khor, H.T. and T.T. Ng, Effects of administration of alpha-tocopherol and tocotrienols on serum lipids and liver HMG CoA reductase activity. Int J Food Sci Nutr, 2000. 51 Suppl: p. S3-11.
131. Qureshi, A.A., et al., Dietary alpha-tocopherol attenuates the impact of gamma-tocotrienol on hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in chickens. J Nutr, 1996. 126(2): p. 389-94.
132. Guthrie, N., et al., Inhibition of proliferation of estrogen receptor-negative MDA-MB-435 and -positive MCF-7 human breast cancer cells by palm oil tocotrienols and tamoxifen, alone and in combination. J Nutr, 1997. 127: p. 544S-548S.
133. Shibata, A., et al., alpha-Tocopherol attenuates the cytotoxic effect of delta-tocotrienol in human colorectal adenocarcinoma cells. Biochem Biophys Res Commun, 2010. 397(2): p. 214-9.
134. Sontag, T.J. and R.S. Parker, Influence of major structural features of tocopherols and tocotrienols on their omega-oxidation by tocopherol-omega-hydroxylase. J Lipid Res, 2007. 48(5): p. 1090-8.
135. Brigelius-Flohe, R., Induction of drug metabolizing enzymes by vitamin E. J Plant Physiol, 2005. 162(7): p. 797-802.
136. Brigelius-Flohe, R., Adverse effects of vitamin E by induction of drug metabolism. Genes Nutr, 2007. 2(3): p. 249-56.
137. Miyamoto, K., et al., Very-high-dose alpha-tocopherol supplementation increases blood pressure and causes possible adverse central nervous system effects in stroke-prone spontaneously hypertensive rats. J Neurosci Res, 2009. 87(2): p. 556-66.
138. Fujita, K., et al., Vitamin E decreases bone mass by stimulating osteoclast fusion. Nat Med, 2012.
139. Klein, E.A., et al., Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA, 2011. 306(14): p. 1549-56.
140. Qureshi, A.A. and H. Mo, Isolation and structural identification of novel tocotrienols from rice bran with hypocholesterolemic, antioxidant and antitumor properties. J Agric Food Chem, 2000(131): p. 223-230.
141. Mensink, R.P., et al., A vitamin E concentrate rich in tocotrienols had no effect on serum lipids, lipoproteins, or platelet function in men with mildly elevated serum lipid concentrations. Am J Clin Nutr, 1999. 69(2): p. 213-9.
142. Mustad, V.A., et al., Supplementation with 3 compositionally different tocotrienol supplements does not improve cardiovascular disease risk factors in men and women with hypercholesterolemia. Am J Clin Nutr, 2002. 76(6): p. 1237-43.
143. Rasool, A.H., et al., Arterial compliance and vitamin E blood levels with a self emulsifying preparation of tocotrienol rich vitamin E. Arch Pharm Res, 2008. 31(9): p. 1212-7.
144. Rasool, A.H., et al., Dose dependent elevation of plasma tocotrienol levels and its effect on arterial compliance, plasma total antioxidant status, and lipid profile in healthy humans supplemented with tocotrienol rich vitamin E. J Nutr Sci Vitaminol (Tokyo), 2006. 52(6): p. 473-8.
145. Tomeo, A.C., et al., Antioxidant effects of tocotrienols in patients with hyperlipidemia and carotid stenosis. Lipids, 1995. 30(12): p. 1179-83.
146. Wahlqvist, M.L., et al., Differential seruresponses to tocopherols and tocotrienols during vitamin E supplementation in hypercholesterolemic individuals without change in coronary risk factors. Nutr Res, 1992. 12: p. S181-S201.
147. Qureshi, A.A., et al., Response of hypercholesterolemic subjects to administration of tocotrienols. Lipids, 1995. 30(12): p. 1171-7.
148. Qureshi, A.A., et al., Suppression of nitric oxide production and cardiovascular risk factor in healthy senior and hypercholesterolemic subjects by a combination of polyphenols and vitamins. J Clin Exp Cardiology, 2012. S5: p. 008.
149. Qureshi, A.A. and D.M. Peterson, The combined effects of novel tocotrienols and lovastatin on lipid metabolism in chickens. Atherosclerosis, 2001. 156(1): p. 39-47.
150. Qureshi, A.A., et al., Novel tocotrienols of rice bran suppress cholesterogenesis in hereditary hypercholesterolemic swine. J Nutr, 2001. 131(2): p. 223-30.
151. Qureshi, A.A., et al., Dietary tocotrienols reduce concentrations of plasma cholesterol, apolipoprotein B, thromboxane B2, and platelet factor 4 in pigs with inherited hyperlipidemias. Am J Clin Nutr, 1991. 53(4 Suppl): p. 1042S-1046S.
152. Qureshi, A.A., et al., Lowering of serum cholesterol in hypercholesterolemic humans by tocotrienols (palmvitee). Am J Clin Nutr, 1991. 53(4 Suppl): p. 1021S-1026S.
153. Qureshi, A.A., et al., delta-Tocotrienol and quercetin reduce serum levels of nitric oxide and lipid parameters in female chickens. Lipids Health Dis, 2011. 10: p. 39.
154. Qureshi, A.A., et al., Synergistic effect of tocotrienol-rich fraction (TRF(25)) of rice bran and lovastatin on lipid parameters in hypercholesterolemic humans. J Nutr Biochem, 2001. 12(6): p. 318-329.
155. Qureshi, A.A., et al., Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF25) of rice bran in hypercholesterolemic humans. Atherosclerosis, 2002. 161(1): p. 199-207.
156. Yu, S.G., et al., Dose-response impact of various tocotrienols on serum lipid parameters in 5-week-old female chickens. Lipids, 2006. 41(5): p. 453-61.
157. McAnally, J.A., et al., Tocotrienols potentiate lovastatin-mediated growth suppression in vitro and in vivo. Exp Biol Med (Maywood), 2007. 232(4): p. 523-31.
158. Hussein, D. and H. Mo, d-Delta-tocotrienol-mediated suppression of the proliferation of human PANC-1, MIA PaCa-2, and BxPC-3 pancreatic carcinoma cells. Pancreas, 2009. 38(4): p. e124-36.
159. Song, B.L. and R.A. DeBose-Boyd, Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme a reductase stimulated by delta- and gamma-tocotrienols. J Biol Chem, 2006. 281(35): p. 25054-61.
160. Parker, R.A., et al., Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem, 1993. 268(15): p. 11230-8.
161. Pearce, B.C., et al., Hypocholesterolemic activity of synthetic and natural tocotrienols. J Med Chem, 1992. 35(20): p. 3595-606.
162. O'Byrne, D., et al., Studies of LDL oxidation following alpha-, gamma-, or delta-tocotrienyl acetate supplementation of hypercholesterolemic humans. Free Radic Biol Med, 2000. 29(9): p. 834-45.
163. Tuyama, A.C. and C.Y. Chang, Non-alcoholic fatty liver disease. J Diabetes, 2012. 4(3): p. 266-80.
164. Magosso, E. Tocotrienols: a novel treatment for non-alcoholic fatty liver disease (NAFLD). in 2nd International Conference on Tocotrienols & Chronic Diseases. 2011. Las Vegas, NV: MPOB.
165. Centers for Disease Control and Prevention. 2009 Age-Adjusted Estimates of the Percentage of Adults with Diagnosed Diabetes. 2012 [cited 2012 August 23].
166. Centers for Disease Control and Prevention. 2009 Age-Adjusted Estimates of the Percentage of Adults Who Are Obese. 2012 [cited 2012 August 23].
167. Qureshi, A.A., et al., Tocotrienols inhibit lipopolysaccharide-induced pro-inflammatory cytokines in macrophages of female mice. Lipids Health Dis, 2011. 9(1): p. 143.
168. Qureshi, A.A., et al., Inhibition of nitric oxide in LPS-stimulated macrophages of young and senescent mice by delta-tocotrienol and quercetin. Lipids Health Dis, 2012. 10: p. 239.
169. Qureshi, A.A., et al., Suppression of nitric oxide induction and pro-inflammatory cytokines by novel proteasome inhibitors in various experimental models. Lipids Health Dis, 2012. 10: p. 177.
170. Viola, V., et al., Why tocotrienols work better: insights into the in vitro anti-cancer mechanism of vitamin E. Genes Nutr, 2012. 7(1): p. 29-41.
171. Pierpaoli, E., et al., Gamma- and delta-tocotrienols exert a more potent anticancer effect than alpha-tocopheryl succinate on breast cancer cell lines irrespective of HER-2/neu expression. Life Sci, 2010. 86(17-18): p. 668-75.
172. American Cancer Society. Cancer Facts & Figures. 2011 [cited; Available from: www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-029771.pdf.
173. Nakagawa, K., et al., In vivo angiogenesis is suppressed by unsaturated vitamin E, tocotrienol. J Nutr, 2007. 137(8): p. 1938-43.
174. Lippman, S.M., et al., Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA, 2009. 301(1): p. 39-51.
175. Campbell, S.E., et al., gamma-Tocotrienol induces growth arrest through a novel pathway with TGFbeta2 in prostate cancer. Free Radic Biol Med, 2011. 50(10): p. 1344-54.
176. Constantinou, C., et al., Induction of caspase-independent programmed cell death by vitamin E natural homologs and synthetic derivatives. Nutr Cancer, 2009. 61(6): p. 864-74.
177. Constantinou, C., et al., Induction of DNA damage and caspase-independent programmed cell death by vitamin E. Nutr Cancer, 2012. 64(1): p. 136-52.
178. Constantinou, C., A. Papas, and A.I. Constantinou, Vitamin E and cancer: An insight into the anticancer activities of vitamin E isomers and analogs. Int J Cancer, 2008. 123(4): p. 739-52.
179. Jiang, Q., et al., Gamma-tocotrienol induces apoptosis and autophagy in prostate cancer cells by increasing intracellular dihydrosphingosine and dihydroceramide. Int J Cancer, 2011.
180. Jiang, Q., et al., gamma-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc Natl Acad Sci U S A, 2004. 101(51): p. 17825-30.
181. Krycer, J.R., L. Phan, and A.J. Brown, A key regulator of cholesterol homoeostasis, SREBP-2, can be targeted in prostate cancer cells with natural products. Biochem J, 2012. 446(2): p. 191-201.
182. Yang, C.S., N. Suh, and A.N. Kong, Does vitamin e prevent or promote cancer? Cancer Prev Res (Phila), 2012. 5(5): p. 701-5.
183. Freeman, M.R. and K.R. Solomon, Cholesterol and benign prostate disease. Differentiation, 2011. 82(4-5): p. 244-52.
184. Kok, D.E., et al., Blood lipid levels and prostate cancer risk; a cohort study. Prostate Cancer Prostatic Dis, 2011. 14(4): p. 340-5.
185. Mondul, A.M., et al., Serum total and HDL cholesterol and risk of prostate cancer. Cancer Causes Control, 2011. 22(11): p. 1545-52.
186. Shafique, K., et al., Cholesterol and the risk of grade-specific prostate cancer incidence: evidence from two large prospective cohort studies with up to 37 years' follow up. BMC Cancer, 2012. 12: p. 25.
187. Murtola, T.J., et al., The Importance of LDL and Cholesterol Metabolism for Prostate Epithelial Cell Growth. PLoS One, 2012. 7(6): p. e39445.
188. Mostaghel, E.A., et al., Impact of circulating cholesterol levels on growth and intratumoral androgen concentration of prostate tumors. PLoS One, 2012. 7(1): p. e30062.
189. Krycer, J.R., L. Phan, and A.J. Brown, A key regulator of cholesterol homeostasis, SREBP-2, can be targeted in prostate cancer cells with natural products. Biochem J, 2012.
190. Zhuang, L., et al., Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest, 2005. 115(4): p. 959-68.
191. Wali, V.B. and P.W. Sylvester, Synergistic antiproliferative effects of gamma-tocotrienol and statin treatment on mammary tumor cells. Lipids, 2007. 42(12): p. 1113-23.
192. Yang, Z., et al., Metabolism of tocotrienols in animals and synergistic inhibitory actions of tocotrienols with atorvastatin in cancer cells. Genes Nutr, 2011.
193. Barve, A., et al., Mixed tocotrienols inhibit prostate carcinogenesis in TRAMP mice. Nutr Cancer, 2010. 62(6): p. 789-94.
194. Husain, K., et al., Vitamin E delta-tocotrienol levels in tumor and pancreatic tissue of mice after oral administration. Pharmacology, 2009. 83(3): p. 157-63.
195. Husain, K., et al., Vitamin E delta-tocotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF-kappaB activation in pancreatic cancer. Mol Cancer Ther, 2011. 10(12): p. 2363-72.
196. Mangialasche, F., et al., Tocopherols and tocotrienols plasma levels are associated with cognitive impairment. Neurobiol Aging, 2012. 33(10): p. 2282-90.
197. Mehat, M.Z., et al., Beneficial effects of vitamin E isomer supplementation on static and dynamic bone histomorphometry parameters in normal male rats. J Bone Miner Metab, 2010. 28(5): p. 503-9.
198. Mohamad, S., et al., Tocotrienol Supplementation Improves Late-Phase Fracture Healing Compared to Alpha-Tocopherol in a Rat Model of Postmenopausal Osteoporosis: A Biomechanical Evaluation. Evid Based Complement Alternat Med, 2012. 2012: p. 372878.
199. Ghosh, S.P., et al., Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int J Radiat Biol, 2009. 85(7): p. 598-606.
200. Li, X.H., et al., Delta-tocotrienol protects mouse and human hematopoietic progenitors from gamma-irradiation through extracellular signal-regulated kinase/mammalian target of rapamycin signaling. Haematologica, 2010. 95(12): p. 1996-2004.
201. Aggarwal, B.B., et al., Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol, 2010. 80(11): p. 1613-31.
202. Levine, S.A., The 21st Century Vitamin E? Delta-Tocotrienol Proves Surprisingly Potent in Fighting Cholesterol, Cardiovascular Disease and Cancer, in In Focus Nutricology Newsletter. June 2008. p. 2-7.
Published in WholeFoods Magazine, October 2012










