Many may be spared from cancer if they are informed of selenium’s role in preventing cancer. Unfortunately, the U.S. Food and Drug Administration (FDA) regulations make it difficult to inform people of this health benefit. The biochemistry of selenium is different from other nutrients, which makes it difficult for many to understand. With all due respect, this apparently includes our good friends at the FDA, who write health claims opinions.
 Many may be spared from cancer if they are informed of selenium’s role in preventing cancer. Unfortunately, the U.S. Food and Drug Administration (FDA) regulations make it difficult to inform people of this health benefit. The biochemistry of selenium is different from other nutrients, which makes it difficult for many to understand. With all due respect, this apparently includes our good friends at the FDA, who write health claims opinions.
Many may be spared from cancer if they are informed of selenium’s role in preventing cancer. Unfortunately, the U.S. Food and Drug Administration (FDA) regulations make it difficult to inform people of this health benefit. The biochemistry of selenium is different from other nutrients, which makes it difficult for many to understand. With all due respect, this apparently includes our good friends at the FDA, who write health claims opinions.
A key point is that although selenium has historically been classified simply as a “mineral,” most of its biological actions are due to the fact that most dietary selenium is converted into the 21st human amino acid (selenocysteine) and thence selenoproteins. Selenocysteine is the active biochemical site within selenoproteins. However, the biochemistry that accomplishes this is unique and has led to misunderstandings about selenium by many (1). Selenoproteins are usually potent antioxidants that can also recycle other antioxidants such as vitamin C and Coenzyme Q10. This is a main factor in my selenium patents (2, 3).
Confounding the problem of “confusing” selenium biochemistry is that the FDA is “confused” about the credible scientific evidence documenting selenium’s benefits. It is not so important that FDA doesn’t quite understand selenium biochemistry, but what is more important is that the FDA is restricting getting the information to the public that selenium may be protective against cancer. In this article, I will discuss 10 of the FDA’s major errors about selenium-containing nutrients and their reduction of cancer risk.
Also, just as important, it appears that the FDA is violating federal court orders regarding how they determine the validity of health claims. This matter has now been taken back to the courts.
In Part 1 of this column (see September 2009 issue), constitutional attorney, Jonathan Emord discussed the unconstitutional abuses of the FDA and the court rulings that were aimed at halting these illegal actions. He also pointed out that in order to protect our constitutional rights, the public must sue the FDA to have the courts force the agency to abide by the laws of the land. As I have stated many times in this column, I strongly support an FDA that protects the people and abides by the laws of our land and the scientific evidence. My goal is to help the FDA help everyone by helping the agency understand the facts and the laws.
As many of you know, I started my laboratory research with selenium more than 50 years ago and began laboratory animal research with selenium and cancer in the 1960s. I have written four books on selenium and I am a co-patentee on selenium nutrients and other selenium compounds. The patents, initiated in 1969, cover antioxidant synergism, selenium and vitamin C synergism, and selenium in the prevention of cancer among other discoveries. They also describe the most effective anticancer compounds. My laboratory animal research began with selenocysteine as the anticancer nutrient and evolved into triphenylphosphine selenide as the most effective anticancer compound.
The vast majority of selenium researchers accept as true that the evidence indicates dietary selenium reduces the incidence of several cancers. . As Drs. Markus Selenius and his colleagues at the world’s premier Karolinska Institute in Sweden recently stated in their latest scientific publication, “It is well established that selenium has cancer preventive effects, and several studies also have shown that it has strong anticancer effects with a selective cytotoxicity on malignant cells while only exerting marginal effects on normal and benign cells” (4).
Selenium compounds are unique in the sense that they may be used both in cancer prevention and treatment (4). The prevention of cancer by selenium compounds is multifaceted, involving a series of biochemical pathways including prevention of mutation via DNA and membrane protection, as well as causing cancer cell death via apoptosis (both G-1 phase and S-phase arrest), phosphorylation of tumor suppressor p53. Various selenium compounds down-regulate survivin and are involved in NF-kB inactivation and thioredoxin reductase activity.
Most selenium researchers are pursuing the roles of the body’s natural selenium-continuing biochemicals called selenoproteins. These selenoproteins are mostly antioxidants that prevent damage to cells that leads to cancer. These antioxidant selenium compounds are moderately and significantly effective in preventing cancer, but they are not the most effective anticancer selenium compounds.
My research, along with that of my colleague David Olson, as well as a small, but rapidly growing, number of others (including Julian Spallholz, Clemet Ip and Howard Ganther, Y. Ohta and Y. Kobayashi, Andrea Mahn and colleagues) focuses on smaller selenium-containing compounds called selenols and their derivatives. Selenols destroy cancer cells at an early stage, primarily through a process of enhanced apoptosis. Thus, these selenol-related compounds are generally more effective in preventing and destroying cancer cells. Recently, Zeng and colleagues have identified seven novel methylselenol responsive genes involved in the regulation of cell cycle and apoptosis and demonstrated that methylselenol inhibits ERK1/2 pathway activation and c-Myc expression (5).
One especially interesting line of research suggests that an important anticancer mechanism of selenium-containing compounds is the down-regulation of surviving, an antiapoptotic protein highly produced in cancer cells that are associated with cancer cell viability (6). Evidence shows that selenium prevents Sp1 binding to its promoter.
In the 1960s, my research indicated it was the selenols and their derivatives that were largely responsible for the marked increase in anticancer activity over other selenium compounds. Drs. Clemet Ip and Howard Ganther have confirmed that the differences in anticancer activity can vary by more than 1,000-fold between selenium compounds (7). All selenium compounds can contribute to selenoprotein formation and thus, cancer prevention, whereas only certain selenium-containing compounds significantly contribute to selenol formation, and thus cancer cell destruction. Methylselenocysteine is one such effective dietary compound, whereas triphenylphosphine selenide is many times more effective. Selenium yeast contains several selenium compounds having moderate to strong anticancer effects.
The FDA does not seem to be aware of the importance of selenols, nor the nuances of selenoprotein formation. Selenium research scientists could not care less about what the FDA thinks about selenium and cancer. These selenium researchers continue to plod away at their research to elucidate the truth. However, we all must protect the truth in the ways we can.
I am pleased to report that a coalition of individuals and companies has taken the step recommended by Attorney Emord in Part 1 and has brought three suits against the FDA involving; 1) selenium qualified health claims, 2) Good Manufacturing Practices and 3) antioxidant qualified health claims.
In the first suit, the plaintiffs seek declaratory and injunctive relief against an FDA final order that violates First Amendment free speech rights and the constitutional mandate of the United States Court of Appeals in Pearson v. Shalala (1999) and Whitaker v. Thompson (2002, 2003).
The suit arises from FDA’s suppression of five qualified health claims for selenium-containing dietary supplements. In an order dated June 19, 2009, the FDA violated our First Amendment rights by completely denying four of our requested qualified health claims and severely restricting a fifth with an inaccurate and highly critical disclaimer. Each of the five requested selenium health claims at issue in this complaint is supported by credible scientific evidence. These claims are not refuted by credible scientific evidence and are not inherently misleading. In its June 19, 2009 order, FDA effectively made near conclusive scientific proof as its standard for allowing any of the requested claims to be communicated to the public. That action directly violates the First Amendment mandates in Whitaker I and the court of Appeals in Pearson I. By censoring the claims in issue, FDA has not permitted accurate representations of nutrition science on the role of selenium in cancer risk reduction to reach the public.
Now, let’s move on to the scientific errors made by the FDA in its consideration of the selenium qualified health claims. The agency made so many preposterous errors regarding both the scientific method and the biochemistry of selenium, that it is almost overwhelming to find a place to start. The 10 most important errors are:
1. The FDA published wrong information in the Federal Register about the strength of the evidence about selenium and cancer.
2. The FDA used the wrong criterion for evaluating qualified health claims.
3. The FDA did not consider the total body of scientific evidence about selenium and cancer.
4. The FDA wrongly states that dietary selenomethionine significantly increases blood selenomethionine levels.
5. The FDA wrongly states that blood selenomethionine level is a biomarker of selenium intake.
6. The FDA wrongly states that blood selenium is mostly selenomethionine.
7. The FDA wrongly states that studies of populations having blood selenium less than 90 micrograms of selenium per liter of blood are not applicable to U.S. citizens.
8. The FDA does not understand the modern classification of selenoproteins.
9. The FDA does not consider selenium levels of subjects or genetic variations in GPX and SOD.
10. The FDA wrongly considers selenomethionine as the most effective anticancer selenium compound and thus, bases the selenium qualified health claims on selenomethionine alone.
FDA Error Number One: Wrong Information Published in the Federal Register
The bias of the FDA against qualified health claims for selenium was apparent from the start when it published wrong information in the Federal Register (8). I pointed out the agency’s error in my public response (9). In its response to Attorney Emord, the FDA ignored its error and did not challenge my discussion of the fact that it had erred (10).

The FDA stated in the Federal Register, “The agency is undertaking a reevaluation of the scientific basis for these authorized health claims and qualified health claims because of new scientific evidence that has emerged for these substance-disease relationships. The new scientific evidence may have the effect of weakening the substance-disease relationship for these authorized health claims…”
The FDA falsely claimed in the Federal Register that the Agency for Healthcare Research and Quality (AHQR) had concluded that the overall strength of the evidence for selenium supplements on cancer was “low,” and thus they intended to reevaluate the scientific evidence and determine if it continues to support the qualified health claim. In fact, the AHQR had correctly pointed out that the overall strength of the evidence for selenium supplements on cancer was “moderate.” Since the AHQR report itself is credible, being it is a report based on research conducted by The Johns Hopkins University Evidence-Based Practice Center under contract to the AHRQ, this report alone is sufficient to establish credible scientific evidence for a qualified health claim.
After review of the available publications, the AHRQ concluded with the following summary of the support for selenium’s anticarcinogenic effects. “Taking into consideration the quantity, quality, and consistency of evidence on the efficacy of selenium in preventing chronic disease, we concluded that the overall strength of evidence is moderate.” I pointed this error out to the FDA in my response in February 2008. The FDA ignored this fact and did not respond to it.
So, the FDA started with an incorrect bias and then proceeded to make critical errors of fact in its response to my public comments. Now, let’s look at the other FDA errors about selenium and cancer.
FDA Error Number Two:Wrong Criterion for Qualified Health Claim
The FDA incorrectly applied the criterion for a full health claim to the petition for a qualified health claim. Basically, the distinction between a “health claim” and a “qualified health claim” is that the former requires essentially conclusive proof whereas the latter requires only “credible scientific evidence.” This is a scientific issue as well as a legal issue.
As Attorney Emord explained in Part 1, “Under Judge Kessler’s standard, if a proposed claim is backed by scientific evidence, FDA may not ban it, regardless of its opinion of the value of the science. Instead, it must allow the claim and, in general, must recite that the evidence is inconclusive. Note that the statement evidence is ‘inconclusive,’ the language recommended by the Court of Appeals is value neutral, yet FDA prefers instead to employ value laden terminology (e.g., the evidence is ‘weak’).
“The qualification FDA uses cannot constitutionally compel a private party to adopt and propound FDA’s bias. Under our First Amendment, the value to be accorded inconclusive science varies based on each person’s perception of its worth. It is the right of each person, not the government, under the First Amendment to assay the relative value of speech offerings, whether those offerings are in matters of politics or science. Under our First Amendment, the government may not condition claim allowance on the speaker’s acceptance and communication of the FDA’s view of the relative worth of the speech.”
FDA Error Number Three: FDA Did Not Consider the Total Body of Science
In July, the Codex Alimentarius Commission adopted new provisions for food supplements, including recommendations on the substantiation of health claims. The adopted Recommendations on the Scientific Substantiation of Health Claims now take into account the totality of the available relevant scientific data and weighing of the evidence for substantiating a health claim, rather than placing primary importance on human intervention trials. It is the total body of scientific evidence that gives scientific meaning to studies, not just a few less-than-perfectly designed intervention studies (clinical trials). It is important to determine if there is a biochemical mechanism that explains how selenium acts to prevent cancer. It is important to determine if selenium prevents various cancers induced by various mechanisms (natural, carcinogen, etc.). It is important to determine if populations low in selenium have more cancer. It is important to determine if cancer patients have lower blood levels of selenium. And, it is also important to determine if adding selenium supplements is protective against cancers.
Selenium has passed all of these criteria. There are well-studied biochemical mechanisms that elucidate several mechanisms through which selenium protects against cancer. There are a plethora of laboratory animal studies demonstrating that selenium-containing nutrients reduce the incidence of both naturally occurring and induced cancers. People with low blood levels of selenium have greater cancer incidence. Cancer patients have lower blood levels of selenium. Giving people selenium-enriched yeast supplements in randomized, placebo-controlled, double-blind clinical studies has reduced cancer incidence. Several clinical trials were described in Part 1. Thus, there is ample credible scientific evidence to support qualified health claims that selenium-containing nutrients reduce cancer risk.
The FDA has erred scientifically in eliminating studies from the total body of evidence just because the people studied were not Americans or may have been undernourished. There are many Chinese and Chinese-Americans who live in the United States and would benefit from selenium supplements. There are many malnourished individuals who would certainly benefit from selenium supplements.
In our 2008 petition, we submitted over 150 peer-reviewed scientific publications supporting the risk-reduction relationship between selenium and cancer (see Docket No. FDA-2008-Q-0323). Additional studies were submitted later. The FDA arbitrarily dismissed all but a small number of these studies and incorrectly labeled them as irrelevant, unreliable, unsupported or inapplicable for a myriad of reasons. The FDA’s arbitrary reasons are not generally accepted as reducing the persuasiveness of the results of the studies in question. As a result of its stringent requirements for publications submitted in support of petitions for health claims, in its final analysis the FDA used only 20 publications out of 233 publications available to make its decision on the claims. The FDA discussed only one intervention report out of 30, and 19 observational reports out of 105. Unfortunately, the FDA scientists and political managers did not subject their analyses to independent peer-review.
The FDA chose to exclude all credible scientific evidence produced by animal and in vitro studies. Although not conclusive evidence, these studies provide credible scientific evidence in support of the evidence that selenium is protective against cancer. In addition, the FDA excluded from its analysis all credible evidence received from both intervention and observational studies conducted in populations that were said to be malnourished, selenium deficient, or had a high prevalence of hepatitis or H. pylori. However, there was no evidence that participants in the studies were malnourished.
The arbitrary nature of consideration of the evidence consequently eliminated much credible scientific evidence from evaluation, which is a major scientific flaw.
FDA Error Number Four: Dietary Selenomethionine Does Not Increase Blood Selenomethionine
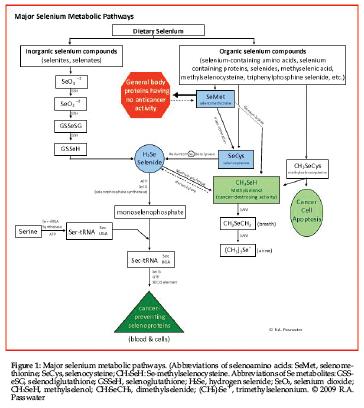
Apparently, and again, with all due respect, the FDA wrongly believes that dietary selenomethionine significantly increases the blood levels of selenomethionine and that the increased blood level of selenomethionine is related to the anticancer action of selenium. This is not so! In reality, much of the dietary selenomethionine (depending on body methionine status) is rapidly incorporated into general body proteins that have no anticancer activity. The remainder of the ingested selenomethionine is rapidly converted into selenocysteine via trans-selenation and circulated in the blood within selenoprotein P or used to form other selenoproteins within the cells (see Figure 1).
FDA Error Number Five: FDA Wrongly States that Selenomethionine Is a Biomarker of Selenium Intake
Selenomethionine incorporated into random body proteins removes selenium from service as an anti- cancer nutrient, rather than contribute to anticancer action as the FDA suggests. The FDA incorrectly states, “However, there are a number of studies to suggest that the metabolism of selenomethionine and other forms of selenium is different, such that the other forms do not raise plasma and serum selenium levels to the same degree as selenomethionine, or not at all. This is because selenomethionine binds to various proteins in the blood (selenoproteins), whereas selenocysteine and inorganic selenium, once absorbed, are transported to the liver, where these compounds enter the selenium metabolic pool and are distributed throughout the body. Therefore, studies that use plasma or serum selenium as a biomarker of intake are considered to reflect the intake of selenomethionine” (page 22, FDA June 19 letter to Emord). What?
The FDA repeats this fallacy on page 35 of the letter. “Assessment of the available scientific evidence suggests that serum and plasma selenium levels reflect intake of selenomethionine, but not intake of selenocysteine or inorganic forms of selenium (e.g., selenate and selenite).”
What? How can the FDA confuse ultimate selenoprotein formation with dietary forms of selenium? The 25 known selenoproteins can be formed under genetic control from any form of selenium that enters the body’s selenium pool. Selenoproteins are defined as proteins in which selenium has been incorporated as selenocysteine via genetic programming. There are two general classes of selenoproteins according to where the selenocysteine is incorporated within the selenoprotein.
Selenoproteins are not just any proteins that happen to contain selenium. Every known human selenoprotein incorporates selenocysteine (and no other form of selenium) within its structure according to genetic control. Selenocysteine is the only genetically encoded amino acid in humans whose biosynthesis occurs on its cognate transfer RNA (tRNA) (1). This process requires multiple features including the selenocysteine insertion sequence element (SECIS), elongation factor EFSec and the binding protein SBP2 (11). Selenocysteine serves as the active site within the known selenoproteins where the selenium atoms serve as redox centers. Figure 1 illustrates this mechanism.
On the other hand, selenomethionine is not incorporated into selenoproteins under genetic control in humans. Instead selenomethionine is randomly incorporated into (not “bound to” or “binds to” as the FDA states) any protein that normally contains methionine as an accidental event. In such cases, the body mistakes selenomethionine for methionine. The FDA even incorrectly states that selenomethionine bound to a protein is a selenoprotein! Today, only proteins that are genetically programmed are classified as selenoproteins and no known human selenoprotein contains or is bound to selenomethionine. Selenomethionine-containing proteins, other than random mistakes, have been found in bacteria, but not in humans.
Dietary selenomethionine is either incorporated into proteins that are not selenoproteins or converted to selenocysteine via trans-selenation or degraded to elemental selenium by beta-lysase and further reduced to selenide. This selenide is then reacted with phosphate from ATP with the help of selenophosphate synthetase (SPS) to form monoselenophosphate. This monoselenophosphate is then added to a serine-based structure that serves as a “backbone” to now form the selenocysteine that is incorporated into selenoproteins as shown in Figure 1.
An extremely important fact is that neither dietary selenocysteine nor selenomethionine is directly incorporated into selenoproteins. All dietary forms of selenium must be metabolized as discussed above for selenomethionine via SPS to yield monoselenophosphate and thence converted to selenocysteine under genetic control to be incorporated into selenoproteins. There is no significant amount of free-form selenomethionine or free-form selenocysteine circulating in the blood, whether ingested as such or produced via SPS. In fact, there is no cellular pool or body pool of selenocysteine. This prevents the unintentional random utilization of selenocysteine that would remove it from the formation of critical selenoproteins.
The forms of selenium that are transported in the blood are primarily selenoproteins including the selenium-transporter selenoprotein P, minor metabolites, degradation products including selenides and selenols and minor selenium-containing compounds such as triphenylphosphine selenide, 3-methylbenzothiazole-2-selenone, isoselenocyanates, tris(methylseleno)methane, allylselenocysteine, methylselenocysteine, and diselenides such as diallyldiselenide.
There are two specific proteins in humans in which selenium is only attached (bound) to the protein portion of the molecules. These two proteins are not classified as selenoproteins. The chemical forms of selenium in the two known examples (14 kDa and 56 kDa) found in liver have not yet been elucidated, but it is known that the TGA codon responsible for selenocysteine incorporation is not involved.
Selenoprotein P function is unproven, but it appears its primary function is to transport selenium in the blood. However, there is evidence that selenoprotein P also acts as an antioxidant. Selenoprotein P is one of two known glycoproteins in plasma and accounts for more than 60% of the plasma selenium level. The other selenium-containing glycoprotein is glutathione peroxidase. Selenoprotein P is very responsive to changes in dietary selenium levels. Selenoprotein P contains up to 10 selenocysteine residues per 43-kDa polypeptide chain, whereas other selenoproteins identified so far have one selenocysteine residue per molecule. A second selenoprotein P of 12-kDa may exist.
Selenophosphate synthetase (SPS) is the product of the SelD gene and is critical for the biosynthesis of known selenoproteins, which contain selenocysteine as their active site. As mentioned earlier, SPS catalyzes the reaction of selenides with AMP to form monoselenophosphate. Monoselenophosphate becomes the selenium donor for the production of the selenocysteine that becomes incorporated into selenoproteins. So far, two selenophosphate synthetases (1 & 2) have been identified: SPS 2, which has selenocysteine as its active site, and SPS 1, which has threonine as its active site.
SPS forms monoselenophosphate (SePO3H3) from ATP and selenide (12). SPS generates selenophosphate from selenite or selenide reacting with a disulfide bond to yield a selenotrisulfide, which then reacts via SPS with phosphate to generate monoselenophosphate.
Now, as to which selenium compounds in the blood accurately reflect dietary intake of selenium, Mahn and colleagues point out that apolipoprotein E, haptoglobin and a-1-antitrypsin respond directly with organic selenium dose (13).
FDA Error Number Six: Blood Selenium Is Not Mostly Selenomethionine
In its June 19 letter to Emord, the FDA admitted an inverse association of blood levels of selenium with cancer risk. Then they incorrectly stated that most of the selenium circulating in the blood was (free-form) selenomethionine and selenocysteine. The FDA stated that “Two chemical forms account for nearly all of the selenium in the plasma and serum components of the blood: selenomethionine and selenocysteine” (Page 21). The fact is selenium in the blood is mostly (60%) contained in Selenoprotein P as bound selenocysteine, and most of the remaining 40% is contained in glutathione peroxidase (also in the form of selenocysteine), with traces of methylselenides being carried to the lungs and kidneys for excretion, plus the trace selenium compounds mentioned earlier. Only an insignificant transitory amount of free selenomethionine can be found in the blood.
As Andrea Mahn and her colleagues state, “Selenium intake has been traditionally quantified as glutathione peroxidase activity or selenium concentration in blood or tissues, however, these indexes do not reflect organic selenium intake” (13) Furthermore, they state, “Selenium chemoprotective capacity seems to have no relation with glutathione peroxidase activity or with residual selenium concentration in tissue.” They report that apolipoprotein E and haptoglobin are biomarkers of metabolic status of organic selenium as they are related to both organic selenium intake and chemoprotective activity, especially genes involved in apoptosis and molecular mechanisms associated with cancer prevention. They also report that transthyretin is a biomarker for methylselenocysteine intake.
Ingested selenium compounds do not persist in the blood for a significant time as they are metabolized in the liver for the purpose of contributing selenium to the body’s selenium pool to be used for the synthesis of genetically programmed selenoproteins. Selenomethionine is an exception because it can be removed prior to metabolism when the body mistakes selenomethionine for methionine in the formation of methionine-containing proteins that are not selenoproteins, nor do they have anticancer activity.
Mechanisms of absorption and metabolism of inorganic and organic selenium differ substantially. Inorganic selenium is recognized by the digestive tissues and is absorbed and converted into selenoproteins (14). In contrast, organic selenium in the form of selenomethionine is not recognized as selenium-containing by mammalian cells (15). As a consequence, selenomethionine is absorbed and metabolized relative to methionine needs. If selenomethionine is broken down within the cell, selenium will be released and recognized by the cell as a mineral, and will be processed according to the need for selenium. However, if the cell does not break down selenomethionine, it will be “accidently” incorporated into a wide variety of proteins that are not genetically programmed to contain selenium.
FDA Error Number Seven: Studies of Populations Having Blood Selenium Less than 90 Micrograms of Selenium per Liter of Blood Are Not Applicable to U.S. Citizens
On page 29 of the June 19 FDA letter to Attorney Emord, the FDA rejects studies in which selenium intake and selenium blood levels are low, arbitrarily claiming that they are not applicable to the general U.S. population. Studies of people having low selenium intakes or low selenium blood levels are indeed appropriate no matter the country or race of individuals studied as the U.S. population consists of people immigrating from or descending from these same countries. The FDA used every excuse it could come up with to throw out as many credible scientific studies as it could.
FDA Error Number Eight: FDA Does Not Understand the Modern Classification of Selenoproteins
The FDA states, “selenomethionine binds to various proteins in the blood (selenoproteins)” (page 22). Once again, selenoproteins are classified as proteins that incorporate selenium only in the form of selenocysteine under genetic control in humans. Selenoproteins are not a random “binding” of selenomethionine and, once again, selenomethionine is NOT a part of selenoproteins.
FDA Error Number Nine: FDA Does Not Consider Blood Selenium Levels (or Other Biomarkers of Organic Selenium Intake) of Subjects or the Genetic Variations in GPX and SOD of Subjects
In nutrition studies, one does not expect to see additional benefits in those who are already adequately nourished. In selenium studies, it is impossible to know the level of selenium nourishment from food tables because the amount of selenium in any food is so variable depending mostly on soil levels of selenium of the area where a crop is grown or animals graze. Therefore, the only reliable measurements of selenium nurtiture are blood selenium levels and/or other biomarkers of organic selenium intake.
In selenium anticancer studies, optimizing the blood selenium levels of those who have low blood selenium levels at the start of the study successfully demonstrate the anticancer effect of selenium-containing nutrients. Feeding insufficient selenium to adequately improve blood selenium levels or feeding additional selenium to raise the blood levels above existing optimal levels of selenium cannot be expected to demonstrate the anticancer effect of selenium.
We cannot expect to see a reduction in cancer risk unless the subjects have less than optimal blood levels of selenium to begin with and they are given a dosage sufficient to raise blood levels to or near the optimal level.
The FDA did not adequately evaluate the blood selenium levels in the few studies they considered, nor did they do so in the many studies they excluded from evaluation.
Another important factor when evaluating the anticancer action of selenium is whether or not the subjects have normal or variant genes for glutathione peroxidase and superoxide dismutase (SOD) production. The metabolic pathways and cancer-protection mechanisms are largely dependent upon the genes for selenoprotein synthesis and selenol formation.
FDA Error Number Ten: Selenomethionine Is Not the Most Effective Anticancer Selenium Compound and thus, Health Claims Should Not Be Based on Selenomethionine Alone
The FDA considers a qualified health claim only for selenomethionine. However, selenomethionine is not as effective an agent against prostate cancer whereas other forms of selenium are. The qualified health claims should apply to ALL dietary forms of selenium, but particularly to selenium-yeast, which has been demonstrated to be effective against cancer in a large clinical trial.
 Selenomethionine is a fine selenium-containing nutrient that provides selenium nourishment and has been shown to have some anticancer properties. However, there are several reasons why selenomethionine is not the compound of choice for cancer prevention, especially prostate cancer prevention. Selenomethionine is the only selenium-containing nutrient that is diverted from cancer prevention by unrelated pathways.
Selenomethionine is a fine selenium-containing nutrient that provides selenium nourishment and has been shown to have some anticancer properties. However, there are several reasons why selenomethionine is not the compound of choice for cancer prevention, especially prostate cancer prevention. Selenomethionine is the only selenium-containing nutrient that is diverted from cancer prevention by unrelated pathways.
Dr. David Waters at Purdue University, using the canine prostate as a scientific model, found that high-selenium yeast was more effective in the reduction of DNA damage of canine prostate cells than selenomethionine (16).
Dr. David McCormick found no effects with selenomethionine supplementation on the prevention of prostate cancer in rats (17). Such negative findings are not the case with other selenium-containing nutrients. Dr. Combs and colleagues have shown that methylselenocysteine was superior to selenomethionine against prostate cancer cells in a dose-dependent inhibition response. Selenomethionine at the same dosage was not cancer growth inhibitory in this study.
Methylselenocysteine is one of the methylselenol precursors found in some selenium yeasts and is also available as a dietary supplement (18).
Yeast incorporate selenium into a variety of compounds, with the predominant compound being selenomethionine, however, the other lesser quantity selenium compounds such as gamma-glutamyl-methylselenocysteine and various selenides and selenols contribute greatly to the anti-cancer effect. It must be stressed that the large study by Dr. Larry and colleagues that showed a 50% reduction in cancer death used selenium-yeast (19).
Selenomethionine is less effective as it is largely incorporated in general body proteins and not available to produce selenoproteins, selenides and selenols. Selenols enhanace apoptosis (G1-phase arrest) via caspase-dependent mechanisms. Selenides lead to apoptosis (S-phase arrest) via caspase independent mechanisms are also involved in phosphorylation of tumor suppressor p53. Various selenium compounds down-regulate survivin and are involved in NF-kB inactivation and thioredoxin reductase activity.
The decision by the FDA to base the qualified health claim for selenium on selenomethionine is totally wrong, unscientific, incomprehensible and ludicrous!
About the “SELECT” Trial
A large study of vitamin E and selenomethionine was prematurely halted in October 2008 (20). This trial has been discussed in recent columns as using the wrong form of selenium and being halted before the selenomethionine had the opportunity to demonstrate its effect (WholeFoods December 2008, WholeFoods April 2009). As mentioned above, selenomethionine is diverted into non-cancer fighting pathways and thus is a less “potent” anti-cancer nutrient and takes longer to demonstrate its beneficial effect. However, some have made the ridiculous conclusion that the premature halting of this trial was evidence that no selenium compound prevents cancer. As the SELECT trial officials who halted the study stated, “Potential limitations of SELECT include that it did not test different formulations or doses of selenium and vitamin E and that it did not definitively assess results in subgroups of men who may have responded differently than did the overall population”(20). In an editorial, Dr. Alan R. Kristal, one of the SELECT researchers said, “The possibility remains that the decisions of SELECT on dose and formulation were wrong”(21).
Several selenium scientists have reported the flaws of the SELECT trial in the peer-reviewed scientific literature. As Dr. Markus Selenius and his group at the Karolinska Institute pointed out in their summary, “Selenium has a clear role in the regulation of normal and malignant cell growth. Despite the disappointing results from the SELECT trial, a great body of evidence suggests that selenium supplementation for a broad public could result in extraordinary health benefits. In fact, the results from the SELECT trial must not lead to the depreciation of all positive and interesting data generated over the past decades. The reported cancer preventive effects in several studies are extraordinary for selenium… In the near future selenium may thus be used by the public as a cancer preventive dietary factor but also be used by the medical profession in the treatment of cancers” (4).
Additional information regarding selenium and cancer may be found on my selenium Web site at www.seleniumresearch.com. WF
Dr. Richard Passwater is the author of more than 40 books and 500 articles on nutrition. He is the
vice president of research and development for Solgar, Inc. Dr. Passwater has been WholeFoods Magazine’s science editor and author of this column since 1984. More information is available on his Web site, www.drpasswater.com.
References
1. S. Palioura, et al., “The Human SepSecS-tRNASec Complex Reveals the Mechanism of Selenocysteine Formation,” Science 325 (5938), 321–325 (2009).
2. R.A. Passwater and D. M. Olson, “Method and Composition to Reduce Cancer Incidence,” U.S. 6,090,414 USPTO. USA, Life Science Labs, Inc. (Minneapolis, MN). “A Continuation-in-Part of Application,” Ser. No. 05/039,142, filed May 20, 1970.
3. R.A. Passwater and D. M. Olson, “Composition Containing Selenium to Reduce Cancer Incidence and Extend Lifespan,” EP 0 750 911 B1. E. P. Office, Life Science Labs, Inc., Minneapolis, MN 55423 (US), 1996.
4. M. Selenius, et al., “Selenium and Selenoproteins in the Treatment and Diagnostics of Cancer,” Antioxid. Redox Signal (2009) Epub Sept. 21, 2009.
5. H. Zeng, et al., (2009). “Methylselenol, A Selenium Metabolite, Induces Cell Cycle Arrest in G1 Phase and Apoptosis via the Extracellular-Regulated Kinase 1/2 Pathway and Other Cancer Signaling Genes,” J. Nutr. 139 (9), 1613–1318 (2009).
6. J.Y. Chun, et al., “Selenium Inhibition of Survivin Expression by Preventing Sp1 Binding to its Promoter,” Mol. Cancer Ther. 6 (9), 2572-2580 (2007).
7. C. Ip, “Lessons from Basic Research in Selenium and Cancer Prevention,” J. Nutr. 128 (11), 1845–1854 (1998).
8. Food and Drug Administration, “Health Claims and Qualified Health Claims; Selenium and Certain Cancers; Reevaluation; Opportunity for Public Comment Docket No. No. FDA-2007-N-0152 (formerly 2007N-0464),” Federal Register 72 (245), 72738–72740 (2007).
9. R.A. Passwater, “Qualified Health Claim: Selenium and Certain Cancers. Response for FDA Notice of Opportunity for Public Comment. FDA Docket No. 2008-Q-0323-0003, FDA Docket No. 2007N-0464 Exhibit II: Passwater Report,” 2008, www.regulations.gov/search/Regs/home.html#documentDetail?R=090000648061021e , www.regulations.gov/fdmspublic/component/main?main=DocketDetail&d=FDA-2008-Q-0323: 52.
10. B.O. Schneeman, “RE: Qualified Health Claim Petition—Selenium and a Reduced Risk of Site-Specific Cancers (FDA-2008-Q-0323),” F. CFSAN, College Park, MD, letter: 39 (2009).
11. L.V. Papp, et al., “From Selenium to Selenoproteins: Synthesis, Identity, and Their Role in Human Health,” Antioxid. Redox. Signal. 9 (7), 775–806 (2007).
12. C.B. Allan, et al. “Responsiveness of Selenoproteins to Dietary Selenium,” Annu. Rev. Nutr. 19: 1–16 (1999).
13. A.V. Mahn, et al., “Organic and Inorganic Selenium Compounds Produce Different Protein Patterns in the Blood Plasma of Rats,” Biol. Res. 42 (2), 163–173 (2009).
14. W.P. Weiss and J. S. Hogan, “Effect of Selenium Source on Selenium Status, Neutrophil Function, and Response to Intramammary Endotoxin Challenge of Dairy Cows,” J. Dairy Sci. 88 (12), 4366–4374 (2005).
15. D. Behne, and A. Kyriakopoulos, “Mammalian Selenium-Containing Proteins,” Annu. Rev. Nutr. 21, 453-473 (2001).
16. D.J. Waters et al., “Effects of Dietary Selenium Supplementation on DNA Damage and Apoptosis in Canine Prostate,” J. Natl. Cancer Inst. 95 (3), 237–241 (2003).
17. D.L. McCormick and K.V. Rao. “Chemoprevention of Hormone-Dependent Prostate Cancer in the Wistar-Unilever Rat,” Eur. Urol. 35 (5–6), 464–467 (1999).
18. G.X. Li, et al., “Superior in vivo Inhibitory Efficacy of Methylseleninic acid against Human Prostate Cancer over Selenomethionine or Selenite,” Carcinogenesis 29 (5), 1005–1012 (2008).
19. L.C. Clark, et al., “Effects of Selenium Supplementation for Cancer Prevention in Patients with Carcinoma of the Skin: A Randomized Controlled Trial, Nutritional Prevention of Cancer Study Group,” JAMA 276 (24), 1957–1963 (1996).
20. S.M. Lippman, et al., “Effect of Selenium and Vitamin E on Risk of Prostate Cancer and other Cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT),” JAMA 301 (1), 39–51 (2009).
21. A.R. Kristal, “Are Clinical Trials the ‘Gold Standard’ for Cancer Prevention Research?” Cancer Epidemiol. Biomarkers Prev. 17, 3289–3291 (2008).
Published in WholeFoods Magazine, Nov. 2009










