A contract manufacturer provides a way to test the waters on a product before committing the resources to in-house implementation. Or they can help improve on an existing formula with updates and an outside perspective.
Let’s take an example to illustrate the point.Health Wright Products (HWP) is a privately held contract manufacturer in a 165,000-square-foot facility in Clackamas, OR. Mark Wright founded the company in 1995. HWP makes encapsulated vitamins and nutritional supplements. It focuses on infusing quality into every capsule—from analyzing raw materials to final product inspection. They know ingredients inside and out, and can help counsel their customers on formulations. One of the things setting HWP apart is that its machines are pharmaceutical grade. They are trying to set a new standard for quality in dietary supplements.
“It starts with the partnership we have with our customers,” Wright explains. “We’re truly an extension of the brand. We look at market data, market research, sales research, and trend analysis for that segment.”
This attention to detail follows throughout the entire process to make sure R&D and product development meet expectations ensuring the consumer is truly getting what the label says they’re getting and at a quality level that will satisfy them so they continue to use the product.
“We bring a level of professionalism but also of proficiency to not only the formulation development and manufacturing, but also the regulatory requirements,” Wright notes.
Differentiation“We’re not just a ‘me-too’ manufacturer,” Wright says, “Most of what we do is with branded supplement companies. Pretty much any retailer you walk into, you’ll find our quality in the brands they carry. We’re the only one I’m aware of that is 100% temperature and humidity controlled from the raw material to blending to encapsulation to packaging. That gives stability to the product throughout its shelf life. We don’t get degradation to the [probiotic] bug count.”
About 25 percent of HWP’s current business is in probiotics. However, the largest number of inquiries it’s getting is for CBD and it expects to enter the CBD market by Q1 of next year with unique relationships established with growers and extractors in Oregon. “What we’re working towards is making sure the supply chain is secure,” he said, noting the company prefers to build the full foundation and platform and have the capability ready and then bring it on the market.
“Our full spectrum organic oils will easily go up against anything coming out of Europe and the ability to customize – to pull certain cannabinoids out” will allow delivery in a liquid filled capsule that preserves the active component from degradation, he says.
“We are fortunate to have Croft Hollingsworth, our chief engineering officer, on board who was instrumental in the development and manufacturing of liquid filled capsules for one of the largest pharmaceutical companies back in 2003,” he adds.
Every product HWP manufactures is unique. “We partner with our customers to understand the product they need to develop and then we create a customized formula. An example is multivitamins: we make hundreds and absolutely no two are alike.”
For quality control, HWP runs checks at every stage. One of its biggest categories is probiotics and companies select them because of their experience, temperature and humidity controlled environment and their ability to custom formulate probiotics for room temperature stability in various packaging configurations. They know how to make sure the “bugs” are produced in an optimal environment.
HWP specializes in capsules and uses the best packaging science available. Whether in glass, plastic, CSP vials or aluminum-aluminum blister cards, the packaging not only stabilizes, but also protects the product. Alu-Alu blister packs offer a moisture and oxygen barrier that provides its own protective cavity and convenience for travelers who just want to throw a 10-count card in their travel bag.
A new pharmaceutical compliant blister pack machine will also be online in Q1 2019. The Bosch Pharmaceutical GKFs, unlike the Capsylon, Zinazi or other China copies, is designed for pharma-grade precision and validation.
From a cleaning standpoint, there are no square edges. The benefits also include data security and cGMP compliant design and construction for the pharmaceutical industry. “HWP makes intentional investments that lead the way for contract manufacturers in the dietary supplement space,” Wright adds. “An example is our investment in electronic record capabilities that will be 21 CFR Part 11 compliant. While it isn’t a requirement, we feel that this is the direction the industry should be moving.”
Today’s consumer is looking for transparency and Part 11 defines the criteria under which electronic records and signatures are considered trustworthy.
The company is also adding liquid fill capabilities with a proprietary capsule inside a capsule that protects against degradation because things that interact are kept completely separate.
“We do a lot of work with the customer upfront so we can help bring these solutions to the market,” Wright adds. “Not only are we making sure we meet the product’s expectation, but also within budget. There’s a market out there for Mercedes, and there’s a market for Kias, and they’re equally important.”
What About CBD?At the recent CBD & Hemp Summit at Expo East, Holly Johnson, PhD, chief science officer for the American Herbal Products Association, laid out just what retailers should ask for to ensure the CBD products they stock are what manufacturers say they are.
While the quality of the product and compliance with 21CFR part 111 are the responsibility of the brand holder (manufacturer), retailers want to be sure they are providing their customers with products they can stand behind.
One idiosyncracy about this market is there is no lab proficiency standard because it’s a “baby evolving industry” with the states responsible and each employs a different standard. “A lot of these materials are being tested by cannabis labs offering different levels of quality,” Johnson noted.
Get the PaperworkAny cannabinoid brand a retailer considers stocking should be able to produce a Certificate of Analysis (COA). Ask for potency and purity levels as well as a High Performance Liquid Chromatography (HPLC) chromatogram. In line with the increased emphasis on transparency, many contain QR codes that link to this information when scanned.
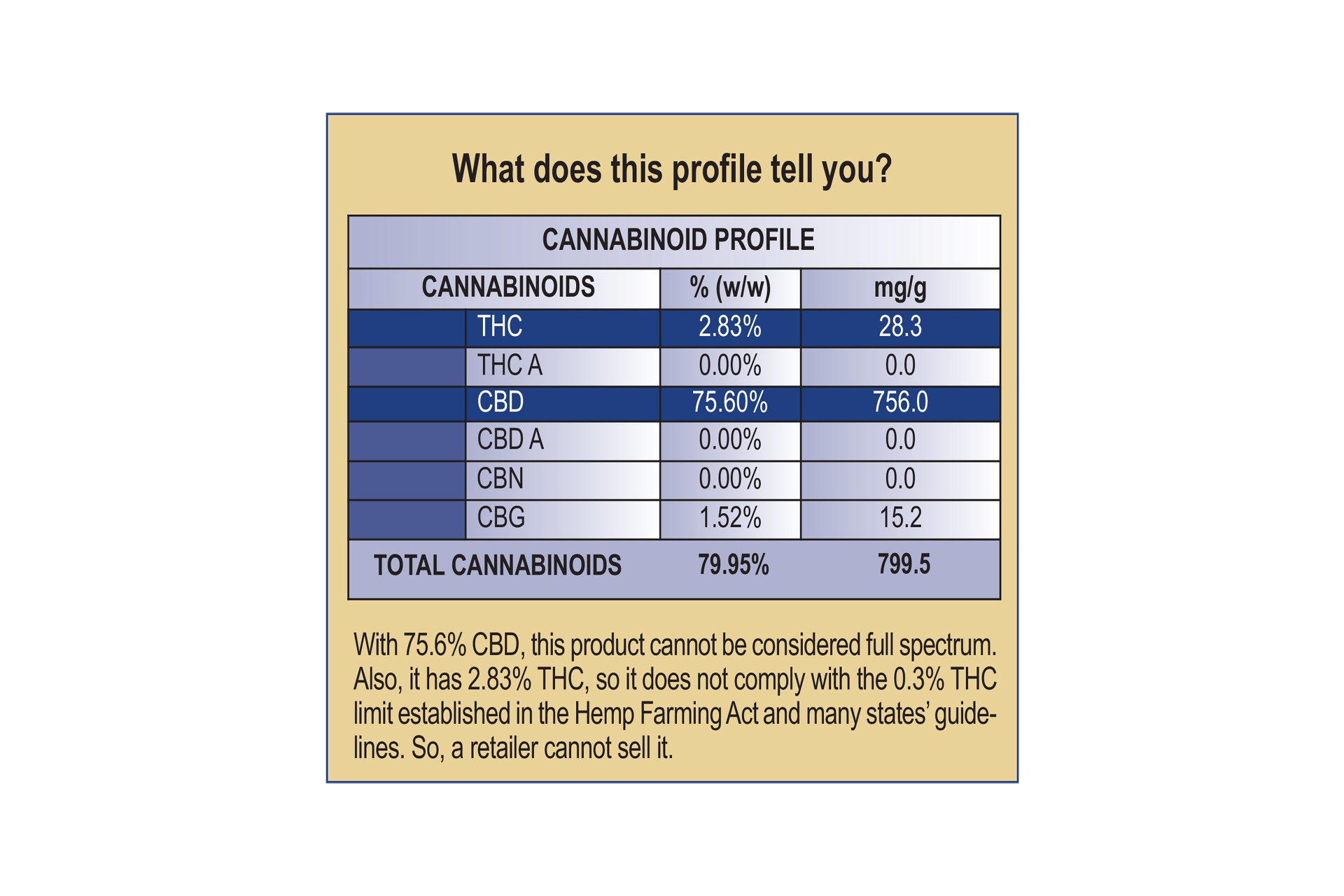
“If a product is purported to be full spectrum, it should contain a range of cannabinoids.”
If a product is purported to be full spectrum, it should contain a range of cannabinoids, not merely CBD, Johnson notes. See Cannabinoid Profile on page 32. An isolate, meanwhile, would be expected to have a concentration of CBD. Of course, THC levels should always be below 0.3%.Once presented with a COA or chromatogram, don’t try to interpret the results without the help of a scientist, Johnson said. Part of her job at AHPA is to act as an “open science hotline” for these types of questions. The Council for Responsible Nutrition offers a similar service.
Two other methods of evaluation, noted Laura Lagano, co-founder of the Holistic Cannabis Academy, are farm source and growing methods as well as extraction methods. Her company offers a cannabis coaching certification program authorized for continuing education credits by the Academy of Nutrition and Dietetics.
As interest increases, the market is growing in so-called “gangapreneurs” who don’t have the sophistication of the dietary supplement industry in terms of understanding how regulation and compliance work.
While natural products retailers are more likely to be dealing with reputable operations, it’s important to be able to explain the differences for customers who might be tempted to buy from the internet or through a multi-level marketing outfit making claims that are not DSHEA-compliant.
 For ManufacturersOf course, as of this writing, the standard for dietary supplements is de-facto unregulated. “If you make a chocolate chip cookie, the FDA can come in and inspect anytime. Put CBD in it and the feds are like, ‘See you later!’,“ Johnson observes.
For ManufacturersOf course, as of this writing, the standard for dietary supplements is de-facto unregulated. “If you make a chocolate chip cookie, the FDA can come in and inspect anytime. Put CBD in it and the feds are like, ‘See you later!’,“ Johnson observes.A manufactuer should act as if the Hemp Farming Act has been passed and you are already under 21CFR part 111. Ask if a lab has been accredited to ISO 17025.
When formulating, be sure to set realistic specs with a “fit for purpose” method. Lab costs are rising rapidly, she said. Budget the money to do the test right.
Be sure your testing is fit for purpose. An oil in a softgel should be easy, but it’s not the same as extracting the CBD in a hard candy or gummy bear.WF










