Tocopherols and tocotrienols have similar chemical structures and have various levels of vitamin E activity. Importantly, tocotrienols provide health benefits beyond those of vitamin E. Both tocopherols and tocotrienols are powerful antioxidants, but tocotrienols also possess powerful neuroprotective, anti-cancer and cholesterol-lowering properties that are not shared by vitamin E in the form of alpha-tocopherol. Tocotrienols and tocopherols are not redundant in function. Unfortunately, many people believe that tocotrienols are just another form of vitamin E and that, since they are already taking vitamin E in their multivitamin, they are covered and mistakenly see no additional benefit in also taking supplemental tocotrienols.
Exciting new research finds that tocotrienols, especially delta-tocotrienol, may be effective against several forms of cancer (including breast cancer, colon cancer, ovarian cancer, pancreatic cancer, and prostate cancer), as well as heart and liver diseases, inflammation, and bone health. We will discuss these studies in Part 2. But first, let’s examine why tocotrienols have some vitamin E activity, but are more than just vitamin E.
Dr. Barrie Tan is hailed as a trailblazer and one of the world’s leading experts on vitamin E. Dr. Tan earned his Ph.D. in Chemistry/Biochemistry from the University of Otago, New Zealand, in 1978 and spent several years as a professor at the University of Massachusetts. Dr. Tan has committed himself to the research and development of phytonutrients that reduce and slow chronic disorders.
Dr. Tan is credited with commercializing forms of vitamin E called tocotrienols from three major sources: Palm, Rice and Annatto. Editor of one prestigious book on tocotrienol and founder of the International Tocotrienol Conference, Dr. Tan was dubbed the “Tocotrienol King” due to his “research background, knowledge, and active involvement in studying tocotrienols.”
Dr. Tan has held roles of Chief Scientific Officer and Scientific Board Member for multinational organizations. His career includes periods of working in association with the US Armed Forces and a Prince of Thailand.
Dr. Tan has successfully launched multiple businesses in the nutrition industry and owns a vast array of patents and intellectual property. He is an internationally sought-after speaker, having presented at several respected conferences in the field including: IFT, ADA, ASN, IHS, A4M, NPA, ICIM, AOCS, IAOMT, and the Academy of Nutrition and Dietetics.
Dr. Tan is currently the President of American River Nutrition, a natural health R&D company he started in 1998.
Passwater: Dr. Tan, we have known each other for decades and have often discussed your research—first with carotenoids stemming from your time at Carotech and then with tocotrienols. Several of our previous chats on tocotrienols are among the most read Vitamin Connection columns in our archives (2-7). Tocotrienols are not an easy area of research, but you have led the path to a better understanding.
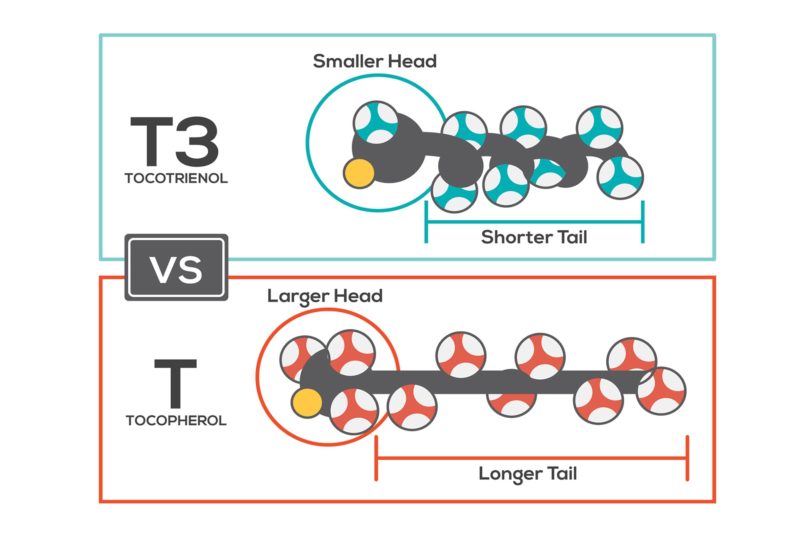
Tocotrienols are still obscure nutrients to many consumers and even health professionals. The very similar chemical structures are deceiving and are responsible for so many thinking that the tocopherols and tocotrienols are essentially the same, while they each have distinct benefits. On paper, the chemical structures look nearly identical, yet the shorter tails of the tocotrienols—due to their unsaturated double bonds—gives them extra mobility, which makes a huge difference in their actions. The greater mobility of tocotrienols in biomembranes allows them to travel much faster (~50 times) than tocopherols to arrest free radicals from lipid oxidation. Dr. Tan, we’ll chat more about this later, if you don’t mind.
Could you give us an overview of what tocotrienols are, and how they were discovered?
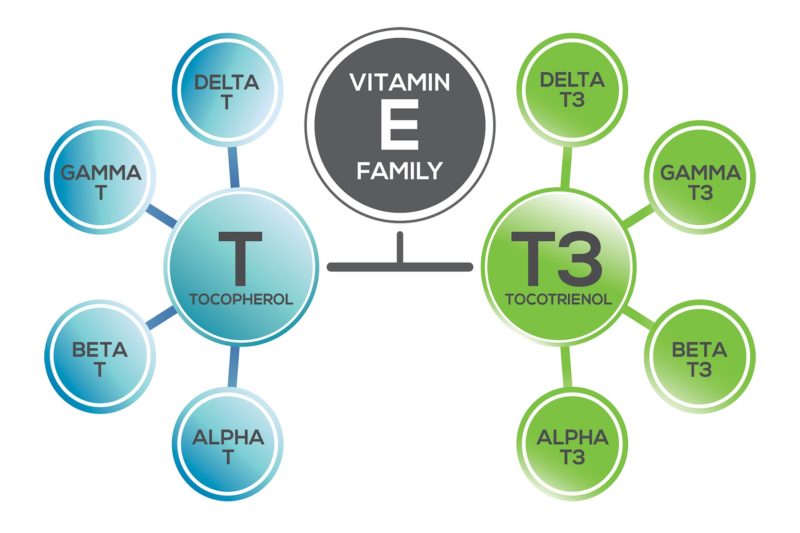
Tan: Vitamin E has a story. As in our lives, stories can be messy. The “Vitamin E story” begins with the discovery of alpha-tocopherol, an extremely closely related chemical structure to the tocotrienols. Tocopherols and tocotrienols are in the same chemical structural family and have several overlapping biochemical actions, as well as several benefits unique to each.
Alpha-tocopherol reached the superstardom of a vitamin (see insert), labeled “E”, because it is needed to bring a fetus to full-term. (Please see Figure 1.)
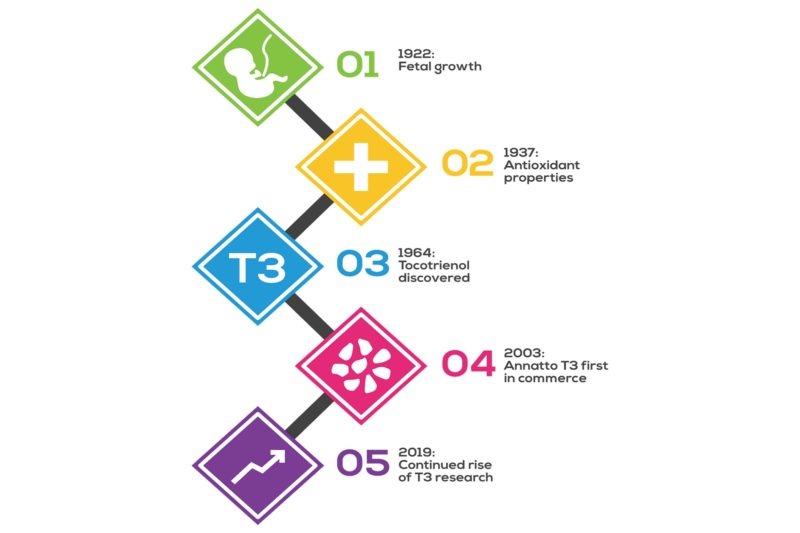
This discovery was made by two UC Berkeley anatomists, Katherine Bishop and Herbert Evans, in 1922 (8). They noted that insufficiency of alpha-tocopherol in animals caused the fetus to be resorbed; this means that if an expectant mother does not have enough vitamin E, she will obtain it from the fetus. To the best of our knowledge, unlike other vitamin deficiencies, this fetal resorption phenomenon has never been documented in humans. Because of the molecular nature of a tocopherol molecule—and tocotrienol molecule—its antioxidant properties were elucidated soon after (1937) (9). These antioxidant properties are compellingly documented such that a unit of measurement, “international unit” (IU), was assigned in 1960 (10). In effect, alpha-tocopherol, had it been discovered as an antioxidant first and fetal resorption second, perhaps it would have been known as an “antioxidant vitamin” rather than a “birth vitamin.” Today, 80+ years later, scientists are still fascinated to publish works on tocopherol and tocotrienol properties in antioxidation, particularly in lipids and lipid membranes. Prevention of fetal resorption remains a faint memory at best, and it’s surprising to most that this was why alpha-tocopherol became a vitamin in the first place!
A generation later, in 1966, tocotrienol came along, with delta-tocotrienol being isolated from rubber latex at the University of Liverpool (11). Tocotrienol structures are so much like tocopherols that they were erroneously named with additional Greek letters epsilon-, phi-, nu-, and zeta-tocopherols. Their official nomenclature was formalized to alpha-, beta-, gamma- and delta-tocotrienols (in an analogous fashion to tocopherols) (See Figure 2) and corrected in the Merck Index in 2001 (12). It took a needlessly long 35 years to see the nomenclature change—it’s not surprising, then, that few knew about the tocotrienol form of vitamin E. At the time, tocotrienols were not studied for fetal resorption and were grouped with tocopherols as antioxidants.
Passwater: Regarding similarity, tocotrienols (in the form of alpha-tocotrienol) have about 25% of the vitamin E activity (expressed in tocopherol equivalents) as alpha-tocopherol. How about the dissimilarities?
Tan: Tocotrienol properties were first differentiated from tocopherol by University of Wisconsin–Madison researchers Asaf Qureshi and Charles Elson (1986) to lower cholesterol (13), an extraordinary ability not shared by alpha-tocopherol.
To understand why tocotrienol’s functions differ from tocopherol’s (the most well-known form of vitamin E), it is useful to look at the compounds’ molecular make-up. The molecular weight of tocotrienol is smaller than the corresponding tocopherol. For example, alpha-tocotrienol is 425 and alpha-tocopherol is 431. On average, a tocotrienol is 1-2% smaller in molecular weight than a tocopherol. The small difference in molecular weight is largely contributed by the sidechains of the distinct molecules. A tocopherol has a longer sidechain and is saturated, meaning without double bonds. A tocotrienol has a shorter sidechain and is unsaturated, meaning with double bonds. Tocotrienol carries the syllable “trienol,” meaning three (“tri”) double bonds (“en”) and an alcohol (“ol”). In nature, antioxidants are ringed alcohols often referred to as phenols. Tocopherols and tocotrienols are phenols and are known antioxidants. How groups are added to rings help in their classification. The ring system can have added substituents (usually methyl groups). Delta-tocotrienol (molecular weight 396) has one methyl in the antioxidant ring and alpha-tocotrienol (molecular weight 425) has three.
Besides the three things that tocopherols and tocotrienols have in common—sharing similar structures, being part of the vitamin E family, and acting as potent lipid antioxidants—they have little else in common pertaining to functions.
Passwater: You just mentioned that both tocopherols and tocotrienols are known antioxidants. How exactly do these antioxidants work in our bodies?A “vitamin” is an essential organic chemical compound that a person needs that:
- he or she cannot make
- must be obtained exogenously (outside the body)
- is required in small amounts
- is essential for normal growth and development
- will cause a disease if absent or insufficient
Here is an oxidation perspective. I find it useful to rise above the noise of the overused term “powerful antioxidant” that has been ascribed to so many nutrients. Let me explain. There are about 38 trillion cells that constitute each person, which is approximately 5,000 times the world population! Let’s make some assumptions that 25% of our weight is fat and that half of this fat is in the form of phospholipids (PL) that compose biomembranes of all our cells in the body. This means that about 8.75 kg (~20 lb.) of phospholipids line cells to contain their constituents—what scientists call organelles. The other half, 20 pounds fat, is stashed away in fat depots in the belly and in fatty organs such as the liver, brain, and muscles. It is the phospholipid fat of bio membranes that is most prone to rancidity and this needs serious oxidative protection. In the early stage of unwanted oxidation, an oxygen is attached to a lipid, called lipid peroxide—peroxide value (PV) is used as measure of omega-3 quality—and this can be arrested with a unique phenolic molecule, such as tocopherol and tocotrienol.
Since we have a mind-boggling 38 trillion cells per person, I would argue that the primary antioxidant protection humans need is cellular lipid membranes—as in phospholipids. I consider lipid membranes to be the “lowest-lying fruit” of oxidative damage.
In the 1980s and 90s, Hermann Esterbauer (University of Graz, Austria) and colleagues looked at what protects lipids in membranes and they studied the cholesterol carriers, including low-density lipoprotein (LDL) (15). The amount of tocopherols dwarf the distant second antioxidant, carotenoids, by 25:1. Therefore, a tocochromanol is perfectly suited to prevent phospholipid oxidation in biomembranes. Hands down!
So for all the antioxidant protection in the human body, protection of lipids is the most important, and a tocochromanol type structure—that of tocopherol and tocotrienol—will best suit the membranes. Such protection is global to all cells because all cells are contained within biomembranes.
Passwater: Which of the tocotrienols holds the greatest promise for overall health benefits?
Tan: Delta-tocotrienol. In many published test-tube and animal studies, the promising direction of potency is delta-tocotrienol ≥ gamma-tocotrienol > alpha-tocotrienol (16-18). Beta-tocotrienol is not abundant in the plant kingdom and is less studied. In clinical studies, many trials used delta-tocotrienol (purified from annatto tocotrienols), gamma-tocotrienol (purified from palm tocopherol-tocotrienol mixtures), delta- and gamma- tocotrienol mixtures (in 90:10 directly from annatto extract), and tocopherol-tocotrienol mixtures (in 25:75 ratio directly from palm extract). These trials have asserted that different tocopherol-tocotrienol mixtures work. However, earlier trials on cholesterol reduction showed that the presence of alpha-tocopherol in mixtures leads to decreased efficacy of tocotrienol (19). Since then, many papers have emerged (especially in the past 5-10 years) covering the entire gamut—test tube, animal and human trials and showing that alpha-tocopherol “puts brakes” on the functions of tocotrienol (18, 20-28).
It has been commented that numerous tocopherol-tocotrienol mixtures (from palm) showed positive results in humans, but they were not compared with tocotrienol alone. Several researchers returned to animal studies and reaffirmed the “brakes” alpha-tocopherol puts on tocotrienol functions, and the evidence indicating this fact has expanded. There are now pharmacokinetic data in trials that showed alpha-tocopherol suppressed the serological bioavailability of tocotrienol (20). For now, animal studies lend compelling alpha-tocopherol antagonism to tocotrienol functions by prohibiting tocotrienol absorption and bioavailability (20, 21, 28), inducing tocotrienol catabolism (breakdown) (27), preventing adipose storage (24), compromising cholesterol and triglyceride reduction (19, 23, 24), exacerbating stroke injury (29), and attenuating cancer inhibition (18, 30).
Here is an example of how to appreciate why there are sometimes positive results in tocopherol-tocotrienol (25:75) trials: Both brake and gas paddles are “on,” and the heavier foot is on the gas paddle, which may allow the car to move forward, but with resistance. Clinical trials on delta-tocotrienol have been conducted to show lowering of lipids including total cholesterol, LDL cholesterol and triglycerides, lowering inflammation, inhibiting pancreatic cancer and resectable pancreatic exocrine neoplasia, improving bone health, reducing non-alcoholic fatty liver disease, and extending the life of advanced stage ovarian cancer patients.
There are numerous ongoing clinical studies in ovarian, lung, colon and breast cancer, and also in obese postmenopausal women. Results of these trials will be forthcoming within the next 2 years. As these trials are designed to prevent potential interference issues, recruitment excludes participants that use alpha-tocopherol as a dietary supplement.
Passwater: Can this delta-tocotrienol or tocotrienols in general be obtained from foods?
Tan: We have very little tocotrienol in the American diet. Typically, tocotrienols can be obtained from foods in small quantities at less than 10 mg/day (31). In our diet, gamma-tocopherol accounts for approximately 50%, alpha tocopherol 20%, and beta- and delta- tocopherol 10% of vitamin E, but all tocotrienols account for only 10%. Tocopherols are often found in vegetable oils such as soy oil, while tocopherol and tocotrienol mixtures are found in palm and rice bran oil, and tocotrienols alone are found in annatto oil. (Please see Figure 4.)
Although in North America, people obtain more gamma-tocopherol from their diet, in Europe the main dietary vitamin E is in the form of alpha-tocopherol. Asian and South American diets may yield greater levels of tocotrienols, because people from these regions use rice bran oil, palm oil and annatto extracts as a regular part of their diet. Tocotrienols can also be found in some processed foods such as potato chips, cookies, Danish pastry, donuts, macaroni, rolls and candy bars (32). In dietary supplements, vitamin E is in the form of mixed tocopherols and usually derived from soy. The tocotrienol levels obtained from foods are generally not sufficient to convey benefits for chronic and aging conditions, and it is therefore recommended to supplement. Unfortunately, most dietary vitamin E supplements provide alpha-tocopherol or mixed tocopherols from soy, and many are semi-synthetic. In these types of supplements, nearly all vitamin E is in the tocopherol form, and tocotrienol is largely absent.
Passwater: If consumers wanted the benefits of delta-tocotrienol for improved health, where would they look for it?
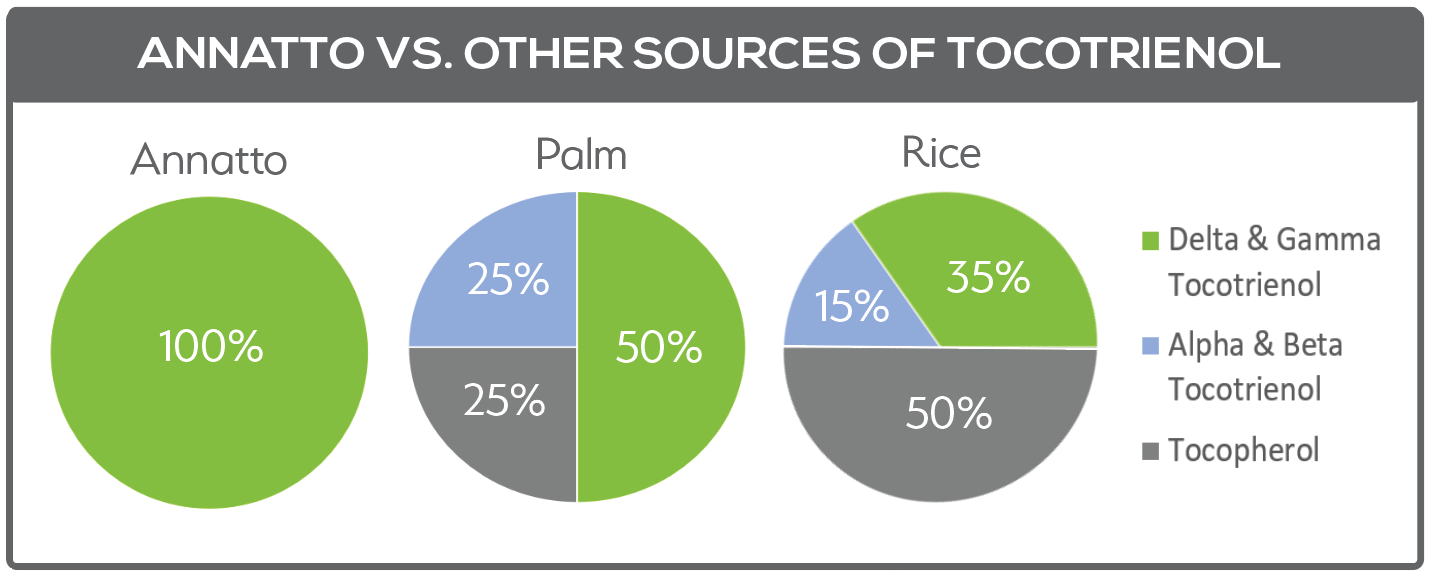
Tan: Tocotrienol supplements are normally derived from annatto, palm or rice. Annatto’s composition is unique: It provides 90% of the potent delta-tocotrienol, whereas palm and rice contain only about 10% of this important isomer. Furthermore, annatto provides exclusively tocotrienols (in the absence of tocopherols), whereas palm and rice contain 25-50% alpha-tocopherol, respectively. Again, this is an important consideration when choosing tocotrienol supplements, because alpha-tocopherol can mute tocotrienol functions. (Please see Figure 5.)

Passwater: Here is an example of one type of a molecule interfering with another from its own structural family. Sometimes “natural mixtures” are best, sometimes they are not. How does this interference manifest itself?
Tan: This is not an isolated occurrence in nature. In our body, even though lutein and beta-carotene as carotenoids have benefits for eye health, taken together, beta-carotene interferes with lutein absorption (33). Alpha-tocopherol and gamma-tocopherol are predominant in nature. However, when taken together, alpha-tocopherol interferes with the absorption of gamma-tocopherol (21). Therefore, naturally occurring mixtures cannot be assumed to be synergistic. Mixed tocopherols are a good marketing idea, but bad absorption science for gamma-tocopherol.
Like I mentioned earlier, not comparing with tocotrienol alone, studies using tocopherol-tocotrienol mixtures (from palm) showed some positive results in humans, but when researchers went back to animal studies they found that alpha-tocopherol put brakes on tocotrienol functions. The mechanism is that a transport protein—alpha-tocopherol transport protein (ATTP)—gives alpha-tocopherol the right-of-passage and facilitates its carriage through the body, thereby inhibiting absorption of tocotrienol.
The first study about the interference of alpha-tocopherol was published in 1996, mentioning that when administered with the lowest alpha-tocopherol concentrations, tocotrienols were more potent to inhibit cholesterol (20). This study concluded that alpha-tocopherol should not be more than 15 percent of the total vitamin E content. In a review of various clinical data, however, we found that this number trends closer to 10 percent, above which alpha-tocopherol begins to pose problems (34).
Passwater: When taken as a supplement, such as from annatto, what is the optimum dose used for various applications?
Tan: For antioxidant benefits and maintenance, 100-150 mg/day would be sufficient. For cardiovascular and anti-inflammatory benefits, the optimum dose is 250 mg/day, based on clinical dose-escalation studies (35, 36). A clinical study in postmenopausal women with osteopenia showed that a daily dose of 300 mg worked well in improving bone turnover and reducing bone loss (37).
Higher doses have been used for more advanced conditions. For example, a phase I dose-escalation trial on delta-tocotrienol in patients with resectable pancreatic cancer showed no adverse effects up to 3,200 mg/day, while apoptosis of cancer cells in patients was observed at the lowest dosage of 200 mg/day (38). It appeared that the optimal dosage for pancreatic cancer was about 600 mg/day. Separately, women with advanced stage ovarian cancer benefited from overall survival by taking 900 mg/day in 3 divided doses (300 mg/day for breakfast, lunch, dinner) (39).
Passwater: Should consumers take any special precautions when using these supplements?
Tan: Tocotrienols should be taken with a meal for absorption and emulsification benefits. This is a natural process. Also, because of interference issues, tocotrienols should be taken about 6 hours apart from alpha-tocopherol if the latter is to be taken as a separate supplement. It is recommended to divide doses greater than 300 mg. Personally, I stopped taking tocopherol some 20 years ago because I believe that the dietary supply of tocopherol is sufficient. There are so many downsides associated with alpha-tocopherol supplementation—flaws that have been repeatedly shown—that I feel like it is not worthwhile to tip-toe around landmines to make it even remotely useful.
Passwater: Dr. Tan, thanks for clarifying so much about tocotrienols for our readers. Let’s take a break and then come back to discuss the recent clinical studies on the health benefits of tocotrienols next month. Specifically, let’s discuss the amazing and powerful studies on inflammation, heart disease, bone health, breast cancer, ovarian cancer, prostate cancer, and pancreatic cancer.
Find part 2 of this interview here.Note: The views and opinions expressed here are those of the author(s) and contributor(s) and do not necessarily reflect those of the publisher and editors of WholeFoods Magazine.References
1. Tan Ph.D., Barrie “The Truth About Vitamin E: The Secret to Thriving with Annatto Tocotrienols” (2019) ISBN 978-1-7338874-2-7 2. Passwater, R.A. Health Benefits Beyond Vitamin E Activity: Solving the Tocotrienol Riddle. An Interview with Dr. Barrie Tan. Whole Foods (June 2008) http://www.drpasswater.com/nutrition_library/tan_1.html 3. Passwater, R.A. Health Benefits Beyond Vitamin E Activity: Solving the Tocotrienol Riddle: Part 2. An Interview with Dr. Barrie Tan Whole Foods (July 2008) http://www.drpasswater.com/nutrition_library/tan_2.html 4. Passwater, R.A. Tocotrienols: Emerging Science and Innovations of Vitamin E Part One: Anti-Cancer Research. An interview with Barrie Tan, Ph.D. Whole Foods (July 2012) https://wholefoodsmagazine.com/columns/vitamin-connection/tocotrienols-emerging-science-and-innovations-vitamin-e-part-one-anti-can/ 5. Passwater, R.A. Tocotrienols: Emerging Science and Innovations of Vitamin E, Part 2. An interview with Barrie Tan, Ph.D. Whole Foods (July 20, 2012) https://wholefoodsmagazine.com/columns/vitamin-connection/tocotrienols-emerging-science-and-innovations-vitamin-e-part-2/ 6. Passwater, R.A. Tocotrienols: Emerging Science and Innovations of Vitamin E, Part 3: Confusing Facts About the Various Forms of Vitamin E and the Role of Tocotrienol. An interview with Barrie Tan, Ph.D. Whole Foods (August 24, 2012) https://wholefoodsmagazine.com/columns/vitamin-connection/tocotrienols-emerging-science-and-innovations-vitamin-e-part-3-confusing/ 7. Passwater, R.A. Tocotrienols: Emerging Science and Innovations of Vitamin E Part 4: A Review of Groundbreaking Tocotrienol Research. An interview with Barrie Tan, Ph.D. Whole Foods (September 18, 2012) https://wholefoodsmagazine.com/columns/vitamin-connection/tocotrienols-emerging-science-and-innovations-vitamin-e-part-4-review-gro/ 8. Evans, H.M. and K.S. Bishop, On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science, 1922. 56: p. 650-651. 9. Olcott, H.S. and O.H. Emerson, Antioxidants and autoxidation of fats: the antioxidant properties of tocopherols. J Am Chem Soc, 1937. 59: p. 1008-1009. 10. Horwitt, M.K., Vitamin E and lipid metabolism in man. Am J Clin Nutr, 1960. 8: p. 451-61. 11. Whittle, K.J., P.J. Dunphy, and J.F. Pennock, The isolation and properties of delta-tocotrienol from Hevea latex. Biochem J, 1966. 100(1): p. 138-45. 12. Merck Index 13th ed., Tocols: 9570 through 9577, 2001, Merck Publishing Group: Rahway, NJ. p. 1693-1694. 13. Qureshi, A.A., et al., The structure of an inhibitor of cholesterol biosynthesis isolated from barley. J Biol Chem, 1986. 261(23): p. 10544-50. 14. Serbinova, E., et al., Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic Biol Med, 1991. 10(5): p. 263-75. 15. Esterbauer, H., et al., The Role of Vitamin E in Lipoprotein Oxidation, in Vitamin E in Health and Disease, L. Packer and C. Fuchs, Editors. 1993, Marcel Dekker: New York. p. 649-671. 16. McIntyre, B.S., et al., Antiproliferative and apoptotic effects of tocopherols and tocotrienols on preneoplastic and neoplastic mouse mammary epithelial cells. Proc Soc Exp Biol Med, 2000. 224(4): p. 292-301. 17. Yu, S.G., et al., Dose-response impact of various tocotrienols on serum lipid parameters in 5-week-old female chickens. Lipids, 2006. 41(5): p. 453-61. 18. Guthrie, N., et al., Inhibition of proliferation of estrogen receptor-negative MDA-MB-435 and -positive MCF-7 human breast cancer cells by palm oil tocotrienols and tamoxifen, alone and in combination. J Nutr, 1997. 127: p. 544S-548S. 19. Qureshi, A.A., et al., Dietary alpha-tocopherol attenuates the impact of gamma-tocotrienol on hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in chickens. J Nutr, 1996. 126(2): p. 389-94. 20. Drotleff, A.M., et al., Human oral bioavailability and pharmacokinetics of tocotrienols from tocotrienol-rich (tocopherol-low) barley oil and palm oil formulations. J Funct Foods, 2014. 7: p. 150-160. 21. Ikeda, S., et al., Dietary alpha-tocopherol decreases alpha-tocotrienol but not gamma-tocotrienol concentration in rats. J Nutr, 2003. 133(2): p. 428-34. 22. Khanna, S., et al., Delivery of orally supplemented alpha-tocotrienol to vital organs of rats and tocopherol-transport protein deficient mice. Free Radic Biol Med, 2005. 39(10): p. 1310-9. 23. Khor, H.T. and T.T. Ng, Effects of administration of alpha-tocopherol and tocotrienols on serum lipids and liver HMG CoA reductase activity. Int J Food Sci Nutr, 2000. 51 Suppl: p. S3-11. 24. Shibata, A., et al., alpha-Tocopherol Attenuates the Triglyceride- and Cholesterol-Lowering Effects of Rice Bran Tocotrienol in Rats Fed a Western Diet. J Agric Food Chem, 2016. 64(26): p. 5361-6. 25. Shibata, A., et al., alpha-Tocopherol attenuates the cytotoxic effect of delta-tocotrienol in human colorectal adenocarcinoma cells. Biochem Biophys Res Commun, 2010. 397(2): p. 214-9. 26. Shibata, A., et al., alpha-Tocopherol suppresses antiangiogenic effect of delta-tocotrienol in human umbilical vein endothelial cells. J Nutr Biochem, 2015. 26(4): p. 345-50. 27. Sontag, T.J. and R.S. Parker, Influence of major structural features of tocopherols and tocotrienols on their omega-oxidation by tocopherol-omega-hydroxylase. J Lipid Res, 2007. 48(5): p. 1090-8. 28. Uchida, T., et al., Tissue distribution of vitamin E metabolites in rats after oral administration of tocopherol or tocotrienol. J Nutr Sci Vitaminol (Tokyo), 2011. 57(5): p. 326-32. 29. Khanna, S., et al., Excessive alpha-tocopherol exacerbates microglial activation and brain injury caused by acute ischemic stroke. Faseb J, 2014. 30. Peralta, E.A., et al., Vitamin E increases biomarkers of estrogen stimulation when taken with tamoxifen. J Surg Res, 2009. 153(1): p. 143-7. 31. Eitenmiller, R. and L. J., Analysis of tocopherols and tocotrienols in food., in Vitamin E: Food Chemistry, Composition, and Analysis2004, Marcel Dekker, Inc.: New York. p. 364-366. 32. Tan, B., Appropriate spectrum vitamin E and new perspectives on desmethyl tocopherols and tocotrienols. JANA, 2005. 8(1): p. 35-42. 33. Kostic, D., W.S. White, and J.A. Olson, Intestinal absorption, serum clearance, and interactions between lutein and beta-carotene when administered to human adults in separate or combined oral doses. Am J Clin Nutr, 1995. 62(3): p. 604-10. 34. Trias, A.M. and B. Tan, Alpha-Tocopherol: A Detriment to Tocotrienol Benefits, in Tocotrienols: Vitamin E Beyond Tocopherols, 2nd ed., B. Tan, R. Watson, and V. Preedy, Editors. 2013, CRC Press: Boca Raton. p. 61-78. 35. Qureshi, A.A., et al., Dose-dependent modulation of lipid parameters, cytokines, and RNA by delta-tocotrienol in hypercholesterolemic subjects restricted to AHA Step-1 diet. Brit J of Med & Med Res, 2015. 6(4): p. 351-366. 36. Qureshi, A.A., et al., Impact of delta-tocotrienol on inflammatory biomarkers and oxidative stress in hypercholesterolemic subjects. Clin. Exp. Cardiology, 2015. 6(4): p. 1000367. 37. Shen, C.L., et al., Tocotrienol supplementation suppressed bone resorption and oxidative stress in postmenopausal osteopenic women: a 12-week randomized double-blinded placebo-controlled trial. Osteoporos Int, 2018. 38. Springett, G.M., et al., A Phase I Safety, Pharmacokinetic, and Pharmacodynamic Presurgical Trial of Vitamin E delta-tocotrienol in Patients with Pancreatic Ductal Neoplasia. EBioMedicine, 2015. 2(12): p. 1987-95. 39. Thomsen, C.B., et al., Delta tocotrienol in recurrent ovarian cancer. A phase II trial. Pharmacol Res, 2019. 141: p. 392-396.










