Dr. Barrie Tan is hailed as a trailblazer and one of the world’s leading experts on vitamin E. Dr. Tan earned his Ph.D. in Chemistry/Biochemistry from the University of Otago, New Zealand, in 1978 and spent several years as a professor at the University of Massachusetts. Dr. Tan has committed himself to the research and development of phytonutrients that reduce and slow chronic disorders. Dr. Tan is credited with commercializing forms of vitamin E called tocotrienols from three major sources—Palm, Rice and Annatto. Dr. Tan has written a new book on tocotrienols, The Truth About Vitamin E: The Secret of Thriving with Annatto Tocotrienols (1). He is the editor of a prestigious technical book on tocotrienols and founder of the International Tocotrienol Conference. Dr. Tan was dubbed the “Tocotrienol King” due to his “research background, knowledge, and active involvement in studying tocotrienols.”
Dr. Tan has held roles of Chief Scientific Officer and Scientific Board Member for multinational organizations. His career includes periods of working in association with the US Armed Forces and the Prince of Thailand.
Dr. Tan has successfully launched multiple businesses in the nutrition industry and owns a vast array of patents and intellectual property. He is an internationally sought-after speaker, having presented at numerous respected conferences in the field including: IFT, ADA, ASN, IHS, A4M, NPA, ICIM, AOCS, IAOMT, and the Academy of Nutrition and Dietetics. Dr. Tan is currently the President of American River Nutrition, a natural health R&D company he started in 1998.
Passwater: Dr. Tan, in Part 1, you mentioned a clinical study on cardiovascular health benefits. Could you review this study with us?
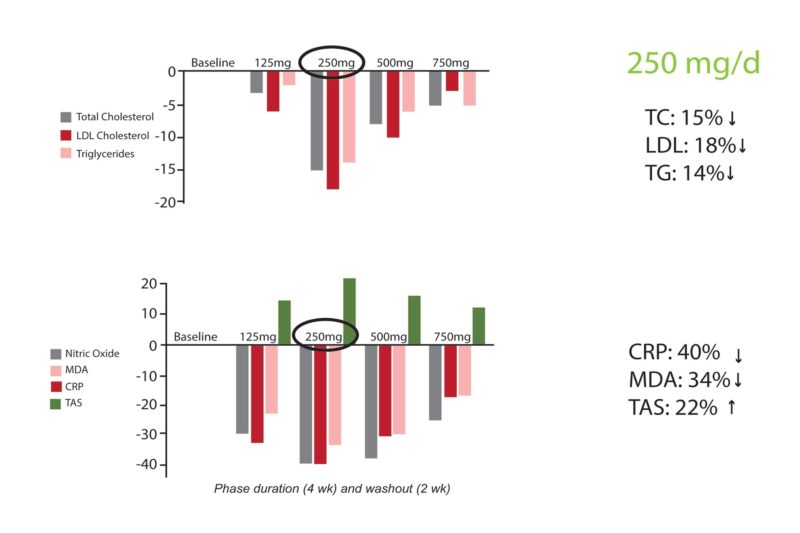
Tan: Certainly. This clinical trial demonstrated compelling evidence about annatto tocotrienols in cardiovascular health benefits (2). Delta-tocotrienol from annatto was used at various doses—125, 250, 500, 750 mg/day in hypercholesterolemic patients. All doses reduced serum lipid parameters, including total cholesterol, LDL cholesterol and triglycerides. The 250 mg daily dose reduced total cholesterol, LDL cholesterol and triglycerides optimally by 15%, 18% and 14%, respectively. The cytokines/biomarkers associated with cardiovascular disease were also down-regulated by 40-60% with annatto tocotrienols (Please see Figure 6). I would go with a 250 mg/d dose for perfect cardiovascular support to maintain lipids and keep inflammation at bay.
Passwater: Interesting. Is the mechanism for this known?
Tan: Tocotrienol’s lipid-lowering effect is related to its structure, where the molecule contains a unique farnesyl moiety that interacts with the human cholesterol biosynthesis pathway (also known as mevalonate or HMG CoA reductase pathway). HMG CoA reductase (HMGR) is the critical enzyme responsible for cholesterol synthesis, and tocotrienol has been found to down-regulate this enzyme and degrade its protein (3, 4). This is particularly true for delta- and gamma-tocotrienol. These tocotrienol isomers act like a sterol, creating a feedback mechanism that leads to the decrease in HMGR activity.
Passwater: Inflammation is closely interconnected with heart disease. Does delta-tocotrienol have anti-inflammatory properties as well?
Tan: Yes! In the early traditional medicine view, patients with heart disease were standardly thought to have elevated cholesterol alone. We now know, however, that half of patients with heart attacks have normal cholesterol levels. Inflammation is a key, with inflammatory cytokines recruiting macrophages, a type of white blood cell, to arterial walls to engulf cholesterol, furthering cardiovascular disease progression and contributing to plaque buildup. At least this is our understanding. Tocotrienol plays a prominent role in the fight against cardiovascular disease and its associated reduction in inflammation. In the clinical study of hypercholesterolemic patients mentioned earlier, the optimum dose of 250 mg/day annatto tocotrienol not only lowered lipid levels, but also reduced inflammation and oxidative stress markers (5). This included a 40% drop in high-sensitivity C-reactive protein (hsCRP) and a 34% decrease in malondialdehyde (MDA), a marker for oxidized fat.
Meanwhile, total antioxidant status (TAS) increased by 22% (5). Furthermore, tocotrienol reduced myocardial injury and inflammation by improving cardiovascular disease-related cytokine gene expression in human plasma, suggesting that it may help improve post-myocardial injury and cardiac muscle recovery, and reduce cardiac fibrosis (scarring of the heart muscle).
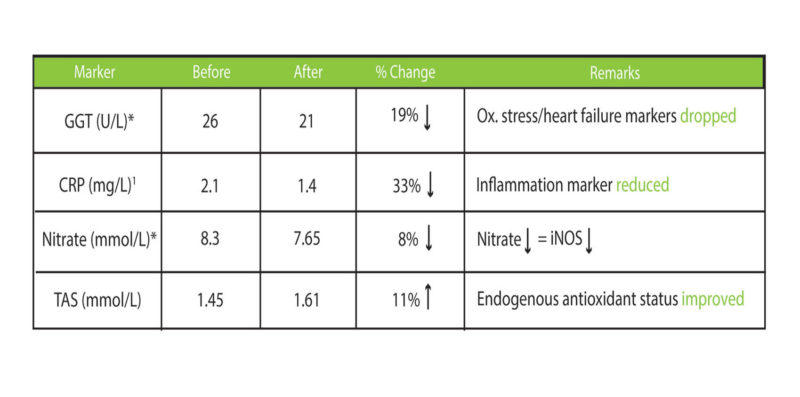
Moreover, in two clinical trials, delta-tocotrienol combined with other anti-inflammatory polyphenols showed synergistic efficacy in both managing dyslipidemia and inhibiting inflammation (6, 7). We further asked if delta-tocotrienol and polyphenols (especially quercetin and resveratrol) would be beneficial to healthy elderly (60-67 years old). We found that the combo decreased oxidative stress and inflammation, while improving antioxidant status (Please see Figure 7). With these data at hand, we now know that a tocotrienol-polyphenol combo is beneficial for the cardiovascularly non-challenged healthy senior.
For combating inflammation, we know that tocotrienols are exceptional at blocking and regulating a variety of inflammatory cytokines, including tumor necrosis factor (TNF), interleukins, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and inducible cyclooxygenase 2 (COX-2).
Passwater: Last year, the American Heart Association and American College of Cardiology announced a new guideline for evaluating blood pressure, lowering the range of blood pressure of what was then considered normal. How about tocotrienol and hypertension?
Tan: Following the new guideline, people being previously considered as high normal or prehypertension are now considered stage 1 hypertension. Tocotrienols, in human studies, have been shown to lower blood pressure and increase arterial compliance (8, 9). In animal studies, tocotrienols lowered blood pressure, and increased total antioxidant activity of plasma and blood vessels, including decreasing their lipid peroxides (10). Gamma- tocotrienol has been shown to decrease systolic blood pressure and enhance nitric oxide synthase activity, which are crucial steps in hypertension development (11). One impressive preclinical study showed that delta-tocotrienol restored kidney functions, which included decreased blood pressure and improved glomerular filtration rate (12). The kidneys’ decrease in oxidative stress (measured via MDA) was matched with an increase in antioxidant function (TAS, superoxide dismutase, catalase, and glutathione).
Passwater: There has also been interesting research with tocotrienols and bone health. Should tocotrienols be included along with nutrients for bone health support?
Tan: Yes! The research on tocotrienol and bone health is an extremely exciting area. According to WHO, about 8 million women in the United States have osteoporosis (severe bone loss) and 22 million women have osteopenia (low bone mass), and hence this research is of enormous importance (13). Osteoporosis causes bone, especially hip, wrist or spine, to become brittle, and can easily cause bone fractures. Although the condition affects all men and women, postmenopausal women are at highest risk. About 50% of postmenopausal women experience osteoporosis-related bone fractures (14). The increased risk may be due to the hormonal changes women experience during menopause, which cause excessive oxidative stress on bone metabolism and escalate the rate of bone resorption over bone formation.
A newly published clinical trial showed how tocopherol-free tocotrienols from annatto benefit and support bone health (15). In the double-blind placebo-controlled study, 89 postmenopausal women with osteopenia were assigned to 3 treatments—placebo, 300 mg annatto tocotrienol per day, or 600 mg annatto tocotrienol per day over the course of 12 weeks. Osteopenia is usually considered to be a precursor to osteoporosis as a condition of bone mineral density lower than normal. The annatto tocotrienol supplementation showed osteo-protective effects in postmenopausal women through increasing bone formation markers by 40-115% (via the BALP/NTX ratio), decreasing bone resorption markers by 7-24% (via the sRANKL/OPG ratio) and reducing oxidative stress by 31-49% (via 8-OHdG).
The anti-osteoporosis benefits by annatto tocotrienol in postmenopausal women were observed when they concurrently took 200IU vitamin D and 500 mg calcium per day. These additional supplements were used by the placebo group as well, which means that the observed bone improvements in postmenopausal women were due to annatto tocotrienol.
Beyond the clinical trial, there is a much larger dossier of pre-clinical research that already supports tocotrienol benefits for the aging bone (16-20).
Passwater: Another published clinical study you mentioned earlier was on pancreatic cancer. Could you share the details of this study with us?
Tan: According to the American Cancer Society, cancer of the pancreas is one of the leading deadly cancers, with 92% of patients dying within 5 years of diagnosis. In 2019, 56,770 people will be diagnosed, and 45,750 will die of pancreatic cancer. Accounting for only 3% of all cancers in the U.S., it is responsible for 7% of cancer deaths (21). Delta-tocotrienol holds huge promise. In a phase I clinical trial, patients with pancreatic cancer received oral delta-tocotrienol from 200 to 3,200 mg per day for 2 weeks prior to surgery (22). All the doses were well tolerated, and no adverse effects or toxicity were reported. The daily 400 to 600 mg doses effectively induced cancer cell apoptosis—programmed cancer cell death—with seemingly greater efficacy with increasing degrees of malignancy. Tumor-grafted studies suggest that delta-tocotrienol inhibits cancer growth, angiogenesis, metastasis, migration, cancer stem cells, and many tumor-related biomarkers (23).
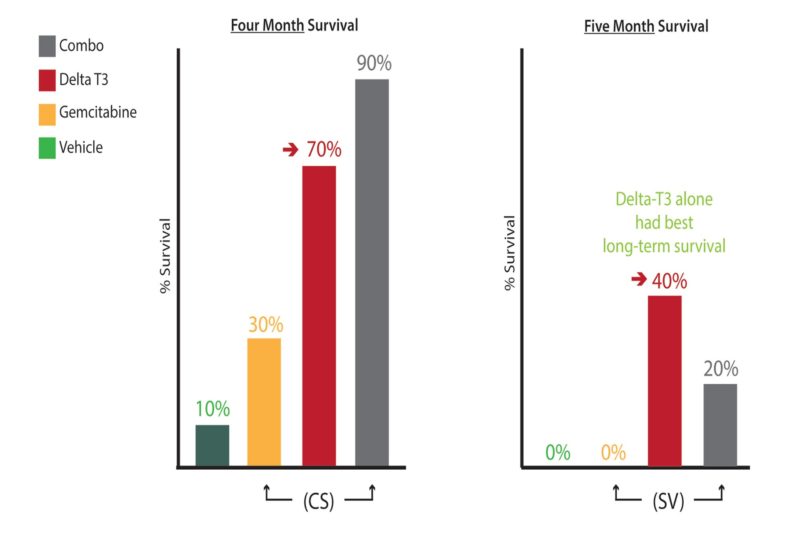
Further, delta-tocotrienol was discovered to synergize with gemcitabine—the only approved chemotherapy drug for pancreatic cancer—to increase survival in a pre-clinical setting, whereas gemcitabine on its own was very toxic (Please see Figure 8) (24, 25). We look forward to seeing this exciting phase I trial move forward to a phase II trial.
Passwater: You also mentioned an ongoing clinical trial on breast cancer. How is delta-tocotrienol expected to work in this context?
Tan: In women, apart from skin cancer, breast cancer is the most common invasive cancer. According to the Breast Cancer Research Foundation, globally, breast cancer represents one in four of all cancers in women (26). Of the various cancers tocotrienol affects, breast cancer was the first to be identified as a target for tocotrienol’s chemopreventive properties. Almost 20 years ago, the ranking of tocotrienol’s effectiveness was shown to be delta-tocotrienol > gamma-tocotrienol > alpha-tocotrienol > tocotrienol-tocopherol mix > delta-tocopherol > alpha-tocopherol for breast cancer (27).
Some breast cancer cells need receptors to chemically bind to hormones to grow, including estrogen receptor (ER), progesterone receptor (PR) and HER-2 receptor. Specific drugs have been developed that can treat breast cancer by blocking corresponding receptors to give a better prognosis. In vitro experiments found that delta-tocotrienol—among all the vitamin Es—had the best molecular fit into the ER protein (28). Moreover, delta-tocotrienol has been shown to inhibit both ER-positive and ER-negative breast cancer cells (29). Interestingly, preclinical studies showed that delta-tocotrienol synergized with tamoxifen—a drug that targets the ER protein—to prevent and treat breast cancer (30).
Here is a red flag: alpha-tocopherol combined with tamoxifen antagonized breast cancer kill and counteracted the drug’s effectiveness (31, 32).
HER-2 positive breast cancer, which has a high incidence of metastasis, disease progression, and chemo resistance, is a severe form of breast cancer with poor prognosis. Cell line and animal studies showed tocotrienol was effective in HER-2 positive and triple-negative (ER, PR, HER-2 receptor negative) breast cancer (33, 34). These preliminary findings led to a phase II clinical trial, which commenced in September 2016 (35).
In this study, HER-2-positive and –negative patients were divided into two groups, with one group receiving standard chemotherapy and the other receiving chemotherapy along with tocotrienol supplementation of 300 mg three times daily. The trial will be completed later this year, and findings are expected to increase our understanding of how tocotrienol works to benefit breast cancer in human patients.
Passwater: Was tocotrienol shown to be effective in prostate cancer as well?
Tan: It was, with delta- and gamma-tocotrienol having a particular inhibitory effect on various prostate cancer cell types. In one study, for example, delta-tocotrienol potently disrupted NFkB signaling and induced programmed prostate cancer cell death (36, 37).
Prostate cancer is one of the most common cancers in men, second only to lung cancer. Although prostate cancer has a high survival rate, one complicating factor is the transitioning from an androgen-dependent to an androgen-independent state of the disease, which carries a poorer prognosis with limited treatment options (38). This is where tocotrienol may help, as it has been shown to reduce androgen-independent prostate cancer cells in addition to lowering PSA levels by 40% (38). When used in combination with gamma-tocopherol, delta-tocotrienol had an additive effect in prostate cancer suppression (38).
One feature associated with poor prognosis in cancer patients is hypoxia, a condition where tumor cells lack oxygen, often leading to increased migration and metastasis. Further confounding cancer therapy is the presence of cancer stem cells, which are the primary mediators of tumor initiation, progression, recurrence, metastasis, and resistance to treatment (39, 40). Amazingly, delta-tocotrienol exhibited a cytotoxic effect on both of these markers, essentially targeting oxygen-deprived prostate cancer stem cells (41). Very recent work underscores the importance of delta- and gamma-tocotrienol as the most potent vitamin Es for prostate cancer (42, 43). I believe these two vitamin E isomers hold tremendous potential for prostate health.
Passwater: Earlier this year, WholeFoods reported beneficial effects of tocotrienol in ovarian cancer. This sounds like a major breakthrough.
Tan: The clinical trial in ovarian cancer patients was groundbreaking, being the first of its kind. The women that participated in this study had been diagnosed with advanced stage ovarian cancer (stages 3 and 4), and had already gone through various available treatment options, but those treatments either didn’t work or the cancer recurred. At this stage of chemotherapy resistance, the goal is to improve the quality and duration of life with few side effects.
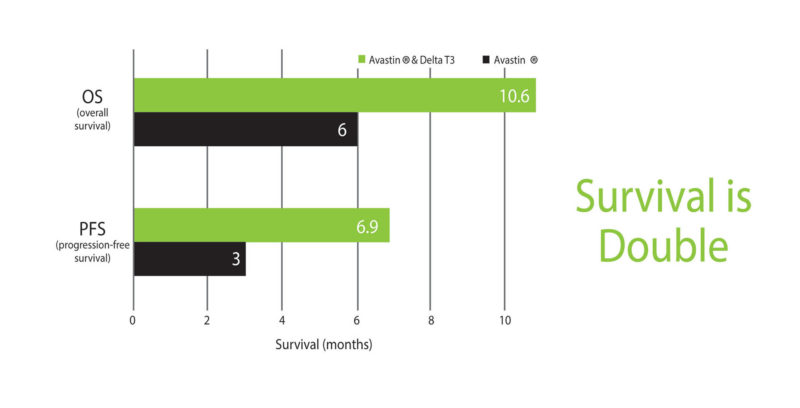
Bevacizumab, commonly known as Avastin, is used as an anti-angiogenic agent for ovarian cancer, and has been known to reduce disease progression by 38%. It was found to reach 25% of disease control in one study, and has a typical progression-free survival duration of 2 to 4 months with overall survival of 5 to 7 months. In the study, carried out at Vejle Hospital in Denmark, a combination of Avastin with tocotrienols nearly doubled survival, with patients reaching progression-free survival of 6.9 months and overall survival of 10.9 months (Please see Figure 9) (44). At 24 months, 25% of the patients were still alive. This is nothing short of a miracle!
Passwater: Those results are incredibly encouraging. Do we know how tocotrienol works to increase survival?
Tan: The researchers noted tocotrienol’s anti-angiogenic properties, which have been confirmed in cell line and animal studies for a variety of cancers (45-50). In cancer, angiogenesis is a pathophysiological process of aberrant and uncontrolled growth of blood vessels emanating from the tumor, which facilitates the delivery of oxygen and nutrients to the tumor. Without angiogenesis, the growth of a tumor beyond a limited size such as 1-2 mm is not possible. To combat cancer, anti-angiogenesis is an excellent target. Tocotrienols essentially starve the tumor cells to death through nutrient deprivation by cutting off these aberrant blood vessels. Professor Teruo Miyazawa was the first to show this for tocotrienol. I first met him at Tufts University in Boston circa 2004 when he showed that tocotrienol was clearly anti-angiogenic (45). He has since documented this phenomenon repeatedly and compellingly, and found that of the vitamin E isomers, delta- tocotrienol was the most potent in inhibiting these unusual new blood vessels to fuel tumor growth (51). Professor Miyazawa proved unequivocally that alpha-tocopherol interferes with tocotrienol’s anti-angiogenic effects (52), reinforcing the importance of supplementing a tocopherol-free tocotrienol.
Passwater: Would tocotrienol have an impact on those undergoing radiation therapy?
Tan: Radiation therapy is a part of cancer therapy to kill malignant tumor cells or prevent tumor recurrence. Dosage levels and duration of radiation treatment will have varying degrees of side effects. Antioxidant supplementation in patients undergoing radiation therapy is an unfounded controversial subject. In general, however, radiation protection is necessary not only for cancer patients, but also for the ordinary American. About one third of our population lives within 50 miles of a nuclear reactor. We have 99 nuclear reactors in the United States, of which 30% are aging. For several years now, the Armed Forces Radiobiology Research Institute (AFRRI), an institution of the U.S. government, has performed extensive research on radiation protection with tocotrienols. AFRRI researchers showed that both delta- and gamma-tocotrienol were potent radiation protectors, possibly due to tocotrienol’s ability to efficiently capture free radicals (53). Beyond antioxidation, both delta- and gamma-tocotrienol also protected the parts of the body first affected by radiation exposure: the bone marrow and blood supply (54). In animal experiments, delta- and gamma-tocotrienol dramatically increased survival of animals exposed to lethal doses of radiation, brought white blood cell and platelet counts back to normal levels, and protected tissues from damage (55-58). AFRRI continues to work with tocotrienols as radiation countermeasures for acute radiation syndrome, and the development for this particularly catastrophic application will involve other DOD agencies down the road (59).
Passwater: All of these are promising research prospects! Is tocotrienol involved in any other notable studies?
Tan: There is ongoing research in various areas of interest, including cognitive wellbeing (60), eye health (61, 62), and specific skin benefits such as wound healing (63).
The most remarkable field of research for tocotrienols, however, is in metabolic syndrome and its associated conditions, including non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH).
Passwater: Tell us more about tocotrienol’s benefits for NAFLD and NASH.
Tan: NAFLD is the 21st century liver disease epidemic (64)! It is affecting 80-100M Americans (~30% adults) (65) and up to 1-in-5 cases of NAFLD transition into the potentially irreversible liver condition NASH (66). The liver is the largest solid organ that performs more than 500 functions every day. Only 30 to 40 years ago, liver cirrhosis was ascribed to alcohol assault, but today it is ascribed to dietary assault. Who would have guessed? The liver is meant to be lean. When it becomes fatty, it gets inflamed and scarred, leading to fibrosis. It also brings on opportunistic infections and cancer to the liver. None of this is good news for our generation and the generations ahead.
Last year, a randomized, double-blind, placebo-controlled clinical trial was published that tested tocotrienol’s benefits in patients with NAFLD (67). The study included 71 individuals, half of which received 600 mg (300 mg twice daily) of annatto tocotrienol for 12 weeks. Tocotrienols significantly improved liver biomarkers, indicating that the stress on the liver was reduced. Triglycerides were lowered by 11%, and lipid oxidative stress marker malondialdehyde and cardiovascular inflammation marker hsCRP were also reduced by 14% and 18%, respectively. When the liver is inflamed, its transaminase enzymes are often elevated. It is generally difficult to reduce these enzymes. Annatto tocotrienol reduced them by about 15%, a notable outcome. Moreover, the Fatty Liver Index (FLI) score—an indicator of intrahepatic fat content—was reduced by 11%, accompanied by an average weight loss of 10lbs. These are all positive impacts on the fatty liver condition.
Various animal studies support these clinical findings (68-72). For example, annatto tocotrienols promoted metabolism by decreasing fat cell size and inflammation in both adipose and liver tissues. A newly published animal trial showed tocotrienol improved glucose tolerance by 15% and decreased serum triglyceride levels by 45% (73).
In adipose tissue, tocotrienol reduced adipocyte size and decreased obesity-related inflammation by preventing macrophage infiltration (inflammation) to adipocytes. Inflammatory quenchers (anti-inflammatory mediators) were increased dose-dependently by ~6-18 fold. Inflammatory agitators (proinflammatory mediators) were decreased by ~2 fold. Furthermore, adipose fatty acid synthesis was decreased by ~60% and fatty acid oxidation was increased by ~14 fold. In liver tissue, tocotrienol supplementation had the lowest number of triglyceride droplets (a measurement of hepatic steatosis) by decreasing fatty acid synthesis by ~40% and elongation by ~45%, and increased fatty acid oxidation by ~125%. As the major lipid catabolism transcription factor, PPARα and PPARδ treated by tocotrienol were increased by 125% and 40%, respectively. Tocotrienol decreased TNFα, a proinflammatory mediator, by ~60%. Overall, annatto tocotrienol supplementation led to an improved metabolic profile (73). These metabolic events are pointing to annatto tocotrienol as an anti-aging nutrient for an aging liver which never stops to perform >500 functions in our body every day!
New clinical trials have already begun, including an obesity trial in postmenopausal women that will examine annatto tocotrienol’s effect on fat mass and visceral adipose tissue, as well as lipid levels and various inflammatory markers (74).
This line of research is extremely important today, especially in light of the rising obesity epidemic. Obesity is on track to replace tobacco use as the major cause of preventable death, and one study determined that obesity led to 47 percent more life years lost than tobacco (75). I am confident that tocotrienols will play a role in alleviating the obesity fallout—associated conditions that include type 2 diabetes, fatty liver, cardiovascular disease, and even cancer—but also think that this complex affliction will require sophisticated prevention strategies and community initiatives to realize a shift in our country’s overall health status.
Passwater: What are some closing thoughts you would like our readers to take home?
Tan: Tocotrienols have been in retail for several decades now, and yet—9 out of 10 times—the everyday consumer you talk to has never even heard of it. Why is that the case? One reason is that—as a silent member of the vitamin E family—tocotrienols have always stood in the shadow of the more popular tocopherols, particularly alpha-tocopherol. Unfortunately, the science behind alpha-tocopherol stands still at fetal development and antioxidant benefits, while some negative reports were picked up by the media that chalked up the entire vitamin E family as a supplement to avoid. At American River Nutrition, we are now working hard to differentiate tocotrienols from tocopherols, and disrupt everyday thinking about vitamin E by broadcasting the results of clinical trials that highlight tocotrienol’s extraordinary functions in chronic and aging conditions. With impressive clinical research surfacing, some have called annatto tocotrienol the new desert island supplement, and for practical purposes, taking 100-150 mg daily is a must (slightly higher doses may be appropriate depending on the application). We have arrived at a tipping point where changing the consumer perspective is critical: Tocotrienol is the 21st century vitamin E.
Passwater: Thank you Dr. Tan for educating our readers about the health benefits of tocotrienols. Readers can find more information about tocotrienols in your new book, The Truth About Vitamin E: The Secret to Thriving with Annatto Tocotrienols and your website at www.barrietan.com (1)Note: The views and opinions expressed here are those of the author(s) and contributor(s) and do not necessarily reflect those of the publisher and editors of WholeFoods Magazine.References
- Tan, B., The Truth About Vitamin E: The Secret to Thriving with Annatto Tocotrienols2019: ISBN 978-1-7338874-2-7.
- Qureshi, A.A., et al., Dose-dependent modulation of lipid parameters, cytokines, and RNA by delta-tocotrienol in hypercholesterolemic subjects restricted to AHA Step-1 diet. Brit J of Med & Med Res, 2015. 6(4): p. 351-366.
- Pearce, B.C., et al., Hypocholesterolemic activity of synthetic and natural tocotrienols. J Med Chem, 1992. 35(20): p. 3595-606.
- Song, B.L. and R.A. DeBose-Boyd, Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme a reductase stimulated by delta- and gamma-tocotrienols. J Biol Chem, 2006. 281(35): p. 25054-61.
- Qureshi, A.A., et al., Impact of delta-tocotrienol on inflammatory biomarkers and oxidative stress in hypercholesterolemic subjects. Clin. Exp. Cardiology, 2015. 6(4): p. 1000367.
- Qureshi, A.A., et al., Suppression of Nitric Oxide Production and Cardiovascular Risk Factors in Healthy Seniors and Hypercholesterolemic Subjects by a Combination of Polyphenols and Vitamins. J Clin Exp Cardiolog, 2012. S5: p. 8.
- Qureshi, A.A., et al., Nutritional Supplement-5 with a Combination of Proteasome Inhibitors (Resveratrol, Quercetin, delta-Tocotrienol) Modulate Age-Associated Biomarkers and Cardiovascular Lipid Parameters in Human Subjects. J Clin Exp Cardiolog, 2013. 4(3).
- Rasool, A.H., et al., Arterial compliance and vitamin E blood levels with a self emulsifying preparation of tocotrienol rich vitamin E. Arch Pharm Res, 2008. 31(9): p. 1212-7.
- Rasool, A.H., et al., Dose dependent elevation of plasma tocotrienol levels and its effect on arterial compliance, plasma total antioxidant status, and lipid profile in healthy humans supplemented with tocotrienol rich vitamin E. J Nutr Sci Vitaminol (Tokyo), 2006. 52(6): p. 473-8.
- Newaz, M.A. and N.N. Nawal, Effect of gamma-tocotrienol on blood pressure, lipid peroxidation and total antioxidant status in spontaneously hypertensive rats (SHR). Clin Exp Hypertens, 1999. 21(8): p. 1297-313.
- Newaz, M.A., et al., Nitric oxide synthase activity in blood vessels of spontaneously hypertensive rats: antioxidant protection by gamma-tocotrienol. J Physiol Pharmacol, 2003. 54(3): p. 319-27.
- Damiano, S., et al., Effects of delta-tocotrienol on ochratoxin A-induced nephrotoxicity in rats. J Cell Physiol, 2018. 233(11): p. 8731-8739.
- National Osteoporosis Foundation, American's bone health: the state of osteoporosis and low bone mass in our nation. Washington DC: National Osteoporosis Foundation, 2002: p. 1-55.
- NIH, Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. JAMA, 2001. 285(6): p. 785-795.
- Shen, C.L., et al., Tocotrienol supplementation suppressed bone resorption and oxidative stress in postmenopausal osteopenic women: a 12-week randomized double-blinded placebo-controlled trial. Osteoporos Int, 2018.
- Chin, K.Y., et al., The Effects of Tocotrienol and Lovastatin Co-Supplementation on Bone Dynamic Histomorphometry and Bone Morphogenetic Protein-2 Expression in Rats with Estrogen Deficiency. Nutrients, 2017. 9(2).
- Chin, K.Y., et al., The Effects of Annatto Tocotrienol on Bone Biomechanical Strength and Bone Calcium Content in an Animal Model of Osteoporosis Due to Testosterone Deficiency. Nutrients, 2016. 8(12).
- Chin, K.Y. and S. Ima-Nirwana, Effects of annatto-derived tocotrienol supplementation on osteoporosis induced by testosterone deficiency in rats. Clin Interv Aging, 2014. 9: p. 1247-59.
- Chin, K.Y. and S. Ima-Nirwana, The biological effects of tocotrienol on bone: a review on evidence from rodent models. Drug Des Devel Ther, 2015. 9: p. 2049-61.
- Shen, C.L., et al., Tocotrienols for bone health: a translational approach. Ann N Y Acad Sci, 2017. 1401(1): p. 150-165.
- American Cancer Society. Key Statistics for Pancreatic Cancer. 2019 6/12/2019]; Available from: https://www.cancer.org/cancer/pancreatic-cancer/about/key-statistics.html.
- Springett, G.M., et al., A Phase I Safety, Pharmacokinetic, and Pharmacodynamic Presurgical Trial of Vitamin E delta-tocotrienol in Patients with Pancreatic Ductal Neoplasia. EBioMedicine, 2015. 2(12): p. 1987-95.
- Husain, K., et al., delta-Tocotrienol, a natural form of vitamin E, inhibits pancreatic cancer stem-like cells and prevents pancreatic cancer metastasis. Oncotarget, 2017. 8(19): p. 31554-31567.
- Husain, K., et al., Prolonged survival and delayed progression of pancreatic intraepithelial neoplasia in LSL-KrasG12D/+;Pdx-1-Cre mice by vitamin E delta-tocotrienol. Carcinogenesis, 2013. 34(4): p. 858-63.
- Husain, K., et al., Vitamin E delta-tocotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF-kappaB activation in pancreatic cancer. Mol Cancer Ther, 2011. 10(12): p. 2363-72.
- Breast Cancer Research Foundation. Breast Cancer Statistics. 2019 6/12/2019]; Available from: https://www.bcrf.org/breast-cancer-statistics.
- McIntyre, B.S., et al., Antiproliferative and apoptotic effects of tocopherols and tocotrienols on normal mouse mammary epithelial cells. Lipids, 2000. 35(2).
- Khallouki, F., et al., Molecular and Biochemical Analysis of the Estrogenic and Proliferative Properties of Vitamin E Compounds. Front Oncol, 2015. 5: p. 287.
- Nesaretnam, K., et al., Tocotrienols inhibit the growth of human breast cancer cells irrespective of estrogen receptor status. Lipids, 1998. 33(5): p. 461-9.
- Guthrie, N., et al., Inhibition of proliferation of estrogen receptor-negative MDA-MB-435 and -positive MCF-7 human breast cancer cells by palm oil tocotrienols and tamoxifen, alone and in combination. J Nutr, 1997. 127: p. 544S-548S.
- Peralta, E.A., et al., Vitamin E increases biomarkers of estrogen stimulation when taken with tamoxifen. J Surg Res, 2009. 153(1): p. 143-7.
- Peralta, E.A., et al., Effect of vitamin E on tamoxifen-treated breast cancer cells. Surgery, 2006. 140(4): p. 607-14; discussion 614-5.
- Pierpaoli, E., et al., Effect of annatto-tocotrienols supplementation on the development of mammary tumors in HER-2/neu transgenic mice. Carcinogenesis, 2013.
- Viola, V., et al., Mitochondrial-dependent anticancer activity of delta-tocotrienol and its synthetic derivatives in HER-2/neu overexpressing breast adenocarcinoma cells. Biofactors, 2013.
- ClinicalTrials.gov. Tocotrienol in Combination With Neoadjuvant Chemotherapy for Women With Breast Cancer (NeoToc). 2019 6/12/2019]; Available from: https://clinicaltrials.gov/ct2/show/results/NCT02909751?view=results.
- Constantinou, C., et al., Induction of caspase-independent programmed cell death by vitamin E natural homologs and synthetic derivatives. Nutr Cancer, 2009. 61(6): p. 864-74.
- Campbell, S.E., et al., gamma-Tocotrienol induces growth arrest through a novel pathway with TGFbeta2 in prostate cancer. Free Radic Biol Med, 2011. 50(10): p. 1344-54.
- Sugahara, R., et al., Annatto Tocotrienol Induces a Cytotoxic Effect on Human Prostate Cancer PC3 Cells via the Simultaneous Inhibition of Src and Stat3. J Nutr Sci Vitaminol (Tokyo), 2015. 61(6): p. 497-501.
- Al-Hajj, M., et al., Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A, 2003. 100(7): p. 3983-8.
- Visvader, J.E. and G.J. Lindeman, Cancer stem cells: current status and evolving complexities. Cell Stem Cell, 2012. 10(6): p. 717-28.
- Kaneko, S., et al., Suppressive Effect of Delta-Tocotrienol on Hypoxia Adaptation of Prostate Cancer Stem-like Cells. Anticancer Res, 2018. 38(3): p. 1391-1399.
- Constantinou, C., C. Charalambous, and D. Kanakis, Vitamin E and cancer: an update on the emerging role of gamma and delta tocotrienols. Eur J Nutr, 2019.
- Fontana, F., et al., delta-Tocotrienol induces apoptosis, involving endoplasmic reticulum stress and autophagy, and paraptosis in prostate cancer cells. Cell Prolif, 2019. 52(3): p. e12576.
- Thomsen, C.B., et al., Delta tocotrienol in recurrent ovarian cancer. A phase II trial. Pharmacol Res, 2019. 141: p. 392-396.
- Miyazawa, T., et al., Anti-angiogenic potential of tocotrienol in vitro. Biochemistry (Mosc), 2004. 69(1): p. 67-9.
- Miyazawa, T., et al., Anti-angiogenic function of tocotrienol. Asia Pac J Clin Nutr, 2008. 17 Suppl 1: p. 253-6.
- Miyazawa, T., et al., Antiangiogenic and anticancer potential of unsaturated vitamin E (tocotrienol). J Nutr Biochem, 2009. 20(2): p. 79-86.
- Nakagawa, K., et al., In vivo angiogenesis is suppressed by unsaturated vitamin E, tocotrienol. J Nutr, 2007. 137(8): p. 1938-43.
- Shibata, A., et al., Tocotrienol inhibits secretion of angiogenic factors from human colorectal adenocarcinoma cells by suppressing hypoxia-inducible factor-1alpha. J Nutr, 2008. 138(11): p. 2136-42.
- Shibata, A., et al., delta-Tocotrienol treatment is more effective against hypoxic tumor cells than normoxic cells: potential implications for cancer therapy. J Nutr Biochem, 2015. 26(8): p. 832-40.
- Shibata, A., et al., Tumor anti-angiogenic effect and mechanism of action of delta-tocotrienol. Biochem Pharmacol, 2008. 76(3): p. 330-9.
- Shibata, A., et al., alpha-Tocopherol suppresses antiangiogenic effect of delta-tocotrienol in human umbilical vein endothelial cells. J Nutr Biochem, 2015. 26(4): p. 345-50.
- Singh, V.K., L.A. Beattie, and T.M. Seed, Vitamin E: tocopherols and tocotrienols as potential radiation countermeasures. J Radiat Res, 2013.
- Satyamitra, M.M., et al., Hematopoietic Recovery and Amelioration of Radiation-Induced Lethality by the Vitamin E Isoform delta-Tocotrienol. Radiat Res, 2011. 175(6): p. 736-45.
- Ghosh, S.P., et al., Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int J Radiat Biol, 2009. 85(7): p. 598-606.
- Kulkarni, S., et al., Gamma-tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation. Radiat Res, 2010. 173(6): p. 738-47.
- Li, X.H., et al., Delta-tocotrienol protects mouse and human hematopoietic progenitors from gamma-irradiation through extracellular signal-regulated kinase/mammalian target of rapamycin signaling. Haematologica, 2010. 95(12): p. 1996-2004.
- Li, X.H., et al., Delta-tocotrienol protects mice from radiation-induced gastrointestinal injury. Radiat Res, 2013. 180(6): p. 649-57.
- Singh, V.K., M. Garcia, and T.M. Seed, A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part II. Countermeasures for limited indications, internalized radionuclides, emesis, late effects, and agents demonstrating efficacy in large animals with or without FDA IND status. Int J Radiat Biol, 2017. 93(9): p. 870-884.
- Fukui, K., et al., Tocotrienols prevent hydrogen peroxide-induced axon and dendrite degeneration in cerebellar granule cells. Free Radic Res, 2012. 46(2): p. 184-93.
- Abdul Nasir, N.A., et al., Reduction of oxidative-nitrosative stress underlies anticataract effect of topically applied tocotrienol in streptozotocin-induced diabetic rats. PLoS One, 2017. 12(3): p. e0174542.
- Abdul Nasir, N.A., et al., Effects of topically applied tocotrienol on cataractogenesis and lens redox status in galactosemic rats. Mol Vis, 2014. 20: p. 822-35.
- Pierpaoli, E., et al., Supplementation with tocotrienols from Bixa orellana improves the in vivo efficacy of daptomycin against methicillin-resistant Staphylococcus aureus in a mouse model of infected wound. Phytomedicine, 2017. 36: p. 50-53.
- Corey, K.E. and L.M. Kaplan, Obesity and liver disease: the epidemic of the twenty-first century. Clin Liver Dis, 2014. 18(1): p. 1-18.
- Mayo Clinic. Nonalcoholic fatty liver disease. 2019 6/18/2019]; Available from: https://www.mayoclinic.org/diseases-conditions/nonalcoholic-fatty-liver-disease/symptoms-causes/syc-20354567.
- Perumpail, B.J., et al., Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol, 2017. 23(47): p. 8263-8276.
- Pervez, M.A., et al., Effects of Delta-tocotrienol Supplementation on Liver Enzymes, Inflammation, Oxidative stress and Hepatic Steatosis in Patients with Nonalcoholic Fatty Liver Disease. Turk J Gastroenterol, 2018. 29(2): p. 170-176.
- Kim, Y., et al., Gamma-tocotrienol attenuates the aberrant lipid mediator production in NLRP3 inflammasome-stimulated macrophages. J Nutr Biochem, 2018. 58: p. 169-177.
- Buckner, T., et al., Annatto Tocotrienol Attenuates NLRP3 Inflammasome Activation in Macrophages. Curr Dev Nutr, 2017. 1(6): p. e000760.
- Kim, Y., et al., Suppression of NLRP3 inflammasome by gamma-tocotrienol ameliorates type 2 diabetes. J Lipid Res, 2016. 57(1): p. 66-76.
- Shibata, A., et al., alpha-Tocopherol Attenuates the Triglyceride- and Cholesterol-Lowering Effects of Rice Bran Tocotrienol in Rats Fed a Western Diet. J Agric Food Chem, 2016. 64(26): p. 5361-6.
- Wong, W.Y., et al., Anti-inflammatory gamma- and delta-tocotrienols improve cardiovascular, liver and metabolic function in diet-induced obese rats. Eur J Nutr, 2017. 56(1): p. 133-150.
- Allen, L., et al., Effects of delta-tocotrienol on obesity-related adipocyte hypertrophy, inflammation and hepatic steatosis in high-fat-fed mice. J Nutr Biochem, 2017. 48: p. 128-137.
- ClinicalTrials.gov. Tocotrienols for Obesity of Postmenopausal Women (Vit E-obesity). 2019 6/12/2019]; Available from: https://clinicaltrials.gov/ct2/show/NCT03705845?term=tocotrienol+texas&rank=1.
- Cleveland Clinic. Obesity is top cause of preventable life-years lost, study shows. 2017 6/12/2019]; Available from: https://www.sciencedaily.com/releases/2017/04/170422101614.htm.










