“The regulated dietary supplement industry supports FDA and FTC using their authorities against any company or individual selling products making COVID-19 cure or prevention claims,” said AHPA President Michael McGuffin in itsannouncement. “While research supports the use of certain herbs and dietary supplements to maintain healthy immune system responses, AHPA is not aware of evidence that would substantiate a claim that any dietary supplement is effective to prevent or treat COVID-19, and such a claim is not allowed under current U.S. law.”
AHPA's analysis, conducted byMerle Zimmermann, Ph.D., AHPA Chief Information Analyst, revealed that, of the 135 warning letters, eight letters were sent by FTC acting on its own and focused on COVID-19 claims in relation to business opportunities.
The bulk of the letters—127 of them—identified one or more products or services as the subject of the letter’s recipient’s claims. The most common subject of the claims in these letters:
- 28 warning letters issued to date involved intravenous therapy.
- 11 letters are directed to claims related to traditional Chinese medicine (TCM) products and therapies
- Essential oil products, colloidal silver products and therapies, herbal products and therapies, homeopathic drug products and therapies, and cannabidoil (CBD) products were also the subject of letters. See Figure 1, which categories 70 warning letter by product or therapy type.
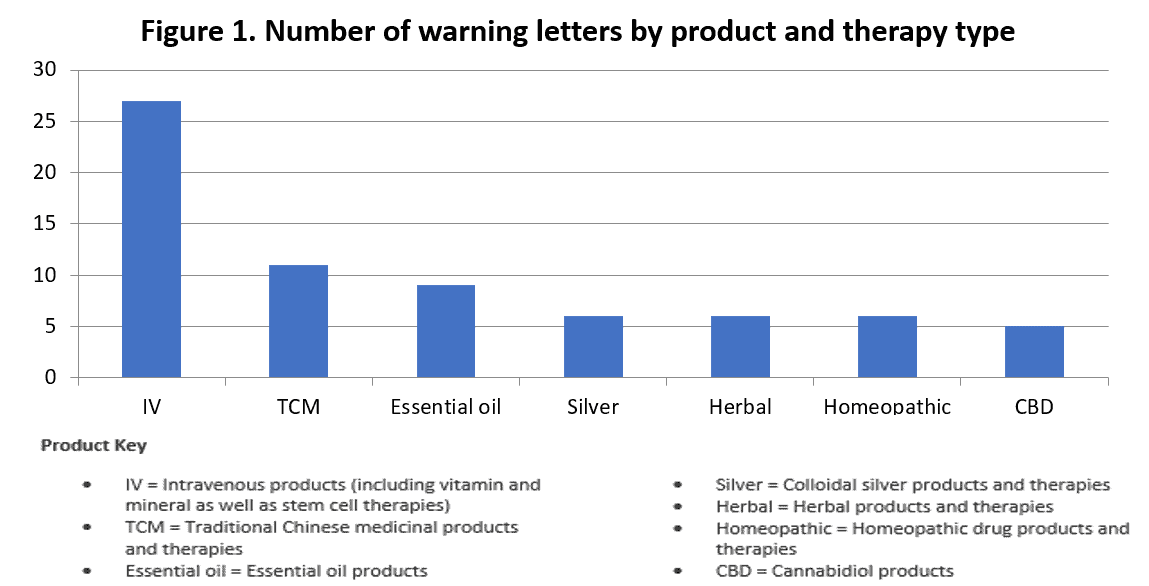
An additional 32 letters ID a single claim subject, and 25 letters deal with multiple categories, such as "viral protection kit," AHPA reported, offering a rundown of the remaining lettershere.
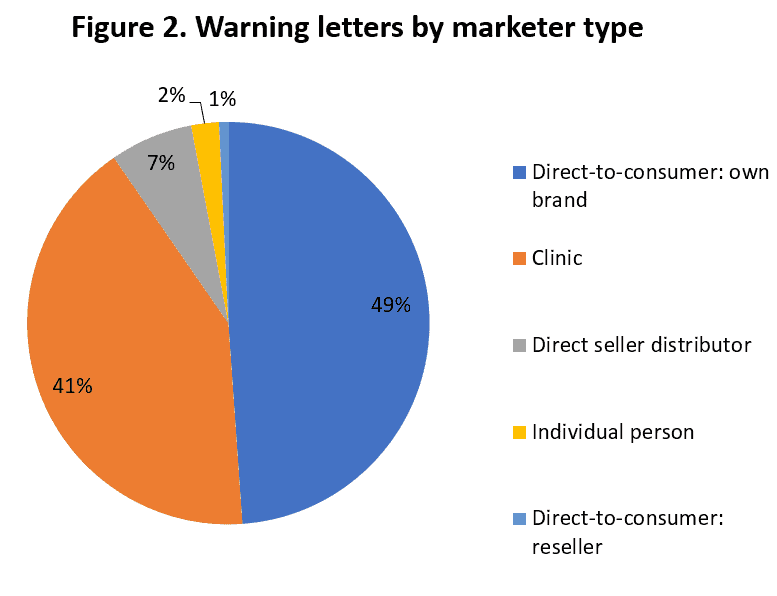
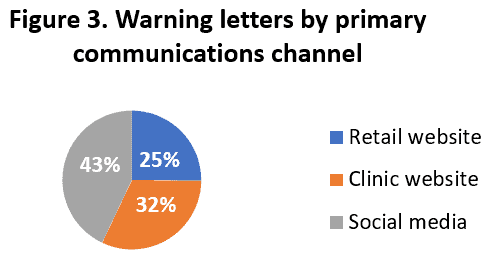
AHPA also analyzed where the claims were originating, and found that claims made by direct-to-consumer marketers, clinics, direct seller distributors, and an occasional individual are receiving the agencies’ attention. (See Figure 2.) Additionally, AHPA determined that often the warning letters were most addressed to claims made on social media, followed by practitioner clinic websites and direct-to-consumer marketers. (See Figure 3.)
AHPA noted that, since the analysis, more letters have been sent. Readherefor limittations and additional details regarding the analysis, andmore resources and educationalopportunities from AHPA.










